The Wound Express System, A Potential Treatment for Patients with Ulcers and Non-Operable Arterial Disease: A Pilot Safety Study
Article information
Abstract
Background
Intermittent pneumatic compression (IPC) devices apply intermittent compression to the thigh, which is thought to facilitate wound healing by improving wound perfusion in patients with chronic limb-threatening ischemia and no option for revascularization. Wound Express is an IPC device but its safety and efficacy are currently unclear. In this study, we aimed to determine the safety of the Wound Express IPC system in this high-risk cohort.
Methods
This was a prospective, non-controlled, non-randomized pilot safety study. In total, 19 patients were screened for inclusion and 10 were recruited. IPC was applied for 2 hours per day for 12 weeks. The primary endpoint was any treatment-related adverse event in the first 3 months. Secondary endpoints included pain using the visual analogue scale, limb status assessed using the Wound, Ischemia, Foot Infection (WIFi) score and quality of life using the Vascular Quality of Life Questionnaire (VASUQOL) score.
Results
Of the 10 patients who were enrolled, eight completed the 12-week course. There were no treatment-related adverse events. One patient died from coronavirus disease 19-related disease and two withdrew for reasons unrelated to treatment. Median pain scores were reduced from 6 to 0, while median VASUQOL increased from 10 to 16.5. WIFi scores were reduced in three out of eight of participants (37.5%).
Conclusion
Wound Express appears to be safe and may decrease pain, improve wound healing and improve quality of life for chronic limb-threatening ischemia patients. Adequately powered, prospectively randomized trials of the Wound Express system to assess its efficacy and safety are warranted.
Introduction
Peripheral vascular disease (PVD) is a wide-ranging disorder, from asymptomatic disease with little or no impact on quality of life, to chronic limb-threatening ischemia (CLTI). CLTI delineates a patient with tissue loss or prolonged pain at rest and is equivalent to Rutherford stage 4 or greater and Fontaine stage 3 or greater [1]. It is estimated that 1 in 2,500 adults in Britain and Ireland are affected by it [2]. Ideally, CLTI patients should be revascularized to alleviate their symptoms, facilitate wound healing and improve their quality of life. Approximately 25% to 40% of patients are not fit enough for surgery, medically unwell or have a disease pattern which is not suitable for reconstruction. Up to 40% of these patients will proceed to have an amputation and their mortality risk is estimated at 20% at 6 months [3]. These patients can be treated conservatively, however medical devices and specialized dressings can also be used [4].
Intermittent pneumatic compression (IPC) can be used as an adjunct to wound healing, for those no-option CLTI patients [5,6]. A systematic review of IPC in CLTI concluded that there was a lack of experimental studies regarding efficacy in patients with no revascularization option or those at risk for amputation. The reviewers commented specifically on two controlled, before and after studies, which showed that IPC may be associated with improved wound healing, limb salvage and pain management. However, they concluded that these studies may have a high risk of bias and suggested well-designed, robust studies before IPC can be recommended for clinical practice [7].
Alvarez et al. [8] evaluated IPC and venous ulcers in 2020. They found that the median time to healing was significantly reduced from 211 to 141 days in those patients who used IPC versus the control group who did not. They also concluded that they experienced less pain and lower limb swelling than those in the control group. Ren et al. [9] also successfully demonstrated that IPC increases foot skin blood flow in both diabetics and non-diabetics.
Wound Express is an IPC system developed to assist in the treatment of chronic wounds by increasing the perfusion to the wound. It is placed on the thigh of the affected side and not directly onto the wound itself, for 2 hours per day. When inflated to 60 mmHg, venous blood is drawn from the wound site. The levels of carbon dioxide and metabolic waste products are reduced, which can accumulate in these areas. Arterial flow subsequently increases and encourages richer flow of blood nutrients and oxygen into the wound bed. The system has been shown to be very effective on venous ulceration, with a small group of arterial, mixed, diabetic, or systemic lupus erythematous-related ulcers also studied [10,11]. However, it has not been tested on patients with severe unreconstructible arterial disease. A visual description of the device can be seen in Supplementary Fig. 1.
We propose to study the effect of this system on wound healing in patients with arterial ulceration who have no option to revascularize their limb by either open or endovascular procedures. In the first instance, we undertook a pilot safety study to establish that the Wound Express system can be safely applied to CLTI patients.
Methods
This was a prospective, non-controlled, non-randomized pilot study. This was due to the small sample size and the availability of 10 devices without charge as it was a pilot safety study. CLTI patients with tissue loss deemed to have unreconstructible disease either due to medical co-morbidities or anatomical reasons (no distal target vessel) were recruited either in the outpatient setting or on the ward. Participants were required to be over 18 years of age, be competent to consent and to have an anticipated survival of at least 6 months. Patients were ineligible if they had been treated with prostaglandins in the previous 3 months, had a mixed etiology ulcer, had failed revascularization in the previous 2 weeks, had an active or previous deep vein thrombosis, had active infection or wet gangrene at the time of screening, had a life expectancy of less than 6 months, were unable to provide consent, were pregnant, had active life-limiting malignancy, had inflow disease too severe to allow safe thigh compression or were felt to be unlikely to comply with the trial protocol.
A member of the team then explained the study fully to the patients who were then asked to sign the consent form. Any questions they had were answered and they were advised they could withdraw their consent at any time.
Participant’s wounds were evaluated clinically and documented using the Wound, Ischemia, Foot Infection (WIFi) criteria [12]. The Vascular Quality of Life Questionnaire (VASUQOL) score was used to assess the impact of the patient’s condition on their life [13]. A visual analogue scale (VAS) score ranging from 0 to 10 was used to assess the pain associated with their ulcer. Baseline ankle-brachial index/toe-brachial index as well as imaging were recorded. The wounds were photographed at baseline. Patients were reviewed in clinic every 3 weeks to assess wound progression.
Participants used the Wound Express (Huntleigh Healthcare Ltd.) system for 2 hours per day for a total of 12 weeks. It was intermittently inflated to 60 mmHg and deflated. They were given a full demonstration and tutorial on application of the device by a study member team and a phone number to ring with any concerns or issues.
The primary objective was to determine the safety profile of the Wound Express system in this high-risk patient cohort. Secondary objectives were to assess what effect the device has on wound progression, pain perceptions of patients, and quality of life. We used the WIFi, VAS, and VASUQOL scores to assess this. The WIFi score was used as an assessment of wound progression, remission, or infection as it is a standardized method of assessing wounds. The VAS score was used as it is an easy way for patients to convey how painful their ulcer is and if the pain has improved or worsened. The VASUQOL score was used as it is an established method of assessing the impact a wound has on the quality of life of patients. The primary safety endpoint was the number of adverse events in the first 3 months of follow-up. Adverse events included worsening of lower limb ischemia including progression of rest pain/ulceration with regards to nature, severity or frequency, the development of clinically significant abnormalities in physical examination or vital signs or occurrence of a local reaction to application of the Wound Express system (pain, oedema, or rash).
The study was reviewed and approved by a Health Service Executive Research Ethics Committee (C.A. 2564). Given the small sample size, it was expected that data would be non-parametric. Continuous outcomes were compared using a MannWhitney U-test. The statistical analysis was undertaken with StatsDirect (StatsDirect v3.3.5). Box and Whisker plots were used to visualize scores pre- and post-intervention.
Results
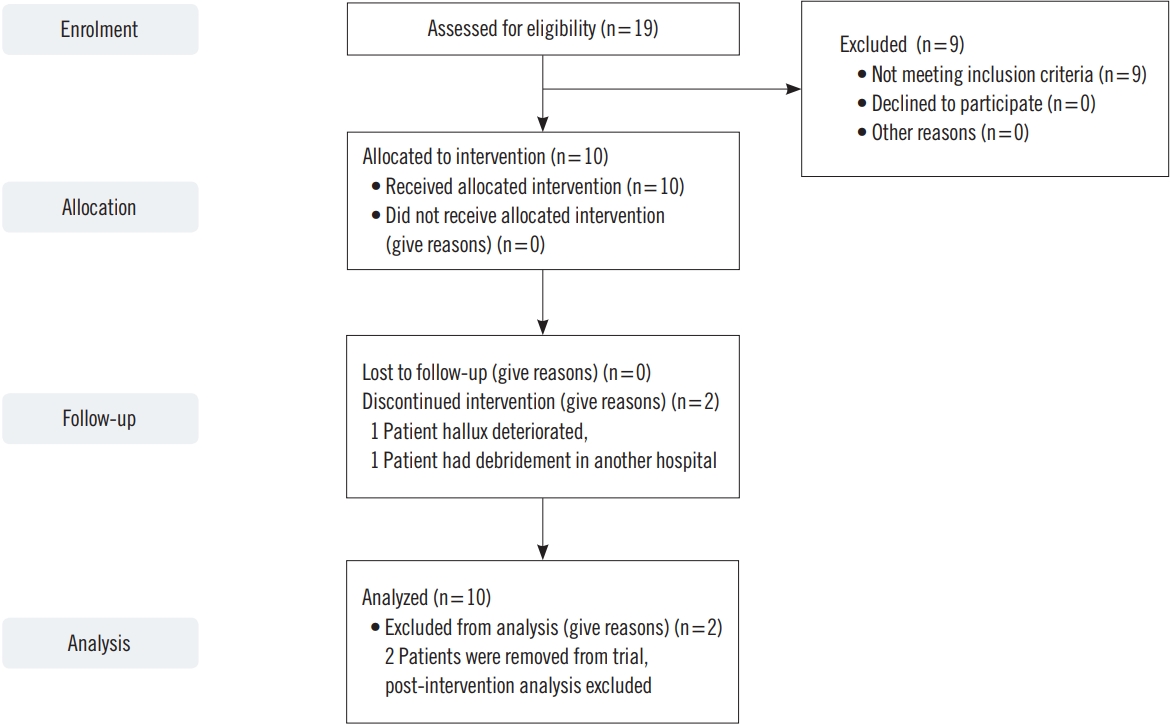
In total, 19 patients were screened for inclusion and 10 patients were recruited. Participants were largely elderly (mean age, 82.7 years; range, 59–95 years) (Fig. 1). Eighty percent were female, 30% diabetic, and 40% had a history of ischemic heart disease (3 previous myocardial infarctions, 4 previous percutaneous coronary intervention). Six patients had attempted revascularization in the past. Patient demographics are summarized in Table 1.
No safety concerns or adverse events related directly to the device were reported. No worsening of ulceration was reported due to use of the device. No local reactions to application of the Wound Express system such as pain, edema or rash were reported.
Of the 10 patients who were enroled, eight completed the 12-week course. One patient died at the end of the trial. They were admitted to hospital and died from coronavirus disease 2019 (COVID-19) related chest complications. Two patients did not complete the trial. The first patient was withdrawn due to deterioration of their hallux ulcer and was admitted for below knee amputation at week 3 review. This wound was severe at enrolment and did not improve. This was thought to be unlikely to be attributed to the device. The second patient was admitted to a tertiary hospital with cellulitis where their wound was surgically debrided resulting in bony exposure. They were admitted for further management. This was likely unrelated to the device.
VAS pain scores
The median pain score at enrolment was 6 (interquartile range, 6–8) (Fig. 2). By 12 weeks, pain scores had decreased to a median value of 0 (interquartile range, 0–6) (P=0.01). Patients were also asked to provide a score for the pain they attributed to use of the device. The median device-related pain score was 0.5 (interquartile range, 0–1.5). No patient reported that the device was too painful to use.

Pain score evaluation. Median visual analogue scale (VAS) pain scores at enrolment and after 12 weeks treatment with Wound Express. The median pain score at enrolment was 6 (interquartile range, 6–8). By 12 weeks, pain scores had decreased significantly to a median value of 0 (interquartile range, 0–6) (P=0.01).
WIFi scores
The median WIFi score at enrolment was 2.5 (interquartile range, 1–4). At 12 weeks, the median WIFi score was unchanged at 2.5 (interquartile range, 1–3.5) (P=0.73) (Fig. 3). Of the eight patients who completed the trial at 12 weeks, three had a reduction in their WIFi score of 1, four had a stable WIFi score but a reduction or improvement in wound description, bar one patient who had compliance issues. One wound initially improved but deteriorated at week 9. The ulceration of the hallux was dry but they developed wet gangrene of the foot dorsum. Their dressing was changed to compression and they continued on the device. At week 12 the wound had dried out again.
VASCUQOL scores
The median VASCUQOL score at enrolment was 10.5 (interquartile range, 8–12.5). At the end of the study the median VASCUQOL score was 16 (interquartile range, 13–20) (Table 2). Of the seven patients who were available to answer the post-intervention questionnaire there was a median increase of 5.5. Of the eight patients who completed the trial, one patient died before the questionnaire could be repeated. This increase suggested that the device may have improved quality of life for some of the participants.
Discussion
Three mechanisms have been put forward to explain the improvements seen in wounds using IPC to help treat CLTI. These mechanisms are: (1) More efficient nitric acid release with increased sheer stress; (2) arresting of autoregulation in peripheral sympathetic autoregulation; and (3) an increase in the arteriovenous pressure gradient [14]. The Irish Health Information and Quality Authority assessed IPC in severe PVD patients who were not candidates for revascularization. They reported that there was a lack of high-quality studies. The data collected suggested IPC may improve limb salvage, wound healing, and pain management. They did not report any serious adverse events related to the use of IPC and concluded that well-designed studies were recommended to reliably evaluate how effective IPC use was in CLTI patients [15]. Ideally, IPC in CLTI should be subjected to a randomized controlled trial comparing best medical therapy (BMT) and wound care plus IPC to BMT alone. Current guidelines from the UK Medical Research Council for evaluating complex interventions recommend the use of feasibility testing in advance of a randomized trial [16]. Therefore, we undertook a pilot safety study of the Wound Express system in no-option CLTI patients to evaluate the safety profile tolerability and acceptability of the device in this patient group.
We demonstrated that the device is safe with regard to adverse events. As mentioned above, one patient died from COVID-19 related respiratory complications and two patients were withdrawn from the trial. One patient was withdrawn after 3 weeks. This patient had severe local tissue loss at recruitment and opted for a below knee amputation at their first review. The second patient presented to another hospital with cellulitis of their leg. They underwent surgical debridement of their dry gangrene there. They presented for routine study review with bony exposure at the debridement site and were withdrawn. Neither of these withdrawals were felt to be attributable to the device. There were no local reactions to the device and patients did not report excessive pain whilst using it. Compliance issues were uncommon, with only a single patient reporting difficulty tolerating the compression device. Overall, the device appears acceptable to the majority of no-option CLTI patients.
The device has also been shown to be cost-effective when treating hard-to-treat venous ulcers. Guest et al. [17] demonstrated that the weekly cost of managing a non-healing wound was £131.61 and an infected wound £224.04. The cost of the garment for the device was £136.50 and the weekly rental cost was £56.00. This represents significant financial savings for the National Health Service. One would assume these savings would be carried over to arterial ulcers. The device also allows for outpatient management of difficult to treat ulcers which again is likely to save money and also resources.
One strength of this study is the prospective nature of it. Robust data was also collected and reviewed. It was the first time this device had been used on exclusively arterial ulcers and was shown to be safe. Although the numbers may have not been powered enough for efficacy outcomes seven of the final eight patients showed potential improvement in wound size and pain scores. It is also novel in this device being used exclusively in arterial disease.
Going forward this device may be used to treat patients with unreconstructible disease. However, another cohort of patients are patients with CLTI but who are at high risk for intervention. Scores such as ERICVA, Prevent III, and Finnvasc have all been used to assist decision-making in this regard [18-20]. If someone is deemed high risk for intervention IPC may be a safe alternative for these patients.
Potential non-severe medical complications of IPC include skin irritation, discomfort, and pain. Rabe et al. [21] constructed an international consensus statement with regards to medical compression in general, including IPC. They listed the aforementioned as the most common complications. Fortunately none of these were reported in this study.
Alvarez et al. [22] also assessed IPC to improve CLTI and PVD with regard to peak walking time. They evaluated the maximum claudication time that their patients could tolerate, and demonstrated an improvement in peak walking time and quality of life with reductions in the size of leg wound area and pain. They also concluded that IPC was safe to use in patients without surgical options for revascularization. This device is placed away from the wound site in the thigh. Other devices are placed closer to the wound. Manfredini et al. [23] reported higher levels of patient compliance using a thigh-applied device versus a foot and calf-applied device.
Kavros et al. [5] studied IPC arterial calf ulcer healing in patients with no revascularization options. They used the ArterialFlow pump system for a minimum of 6 hours per day. At 18 months, their results demonstrated that 58% of wounds had healed fully using the pump system compared to only 17% in the control group who did not use it. Furthermore, the rate of below knee amputation was reduced from 83% to 42%.
Pawlaczyk et al. [6] also evaluated the use of IPC in patients who had either undergone revascularization or conservative management to assess the effect on skin flow normalization. Fifty-seven out of 116 patients underwent postoperative IPC. Thirty-seven underwent either open or endovascular treatment and 20 were managed conservatively. The parameters they investigated included microcirculation flow, skin blood flow, and transcutaneous pressure of oxygen pre- and post-intervention/conservative management and at regular intervals for up to 90 days. They all underwent 14 days of IPC from postoperative day 1, lasting 1 hour per day. The groups who underwent IPC showed significantly reduced edema at nearly all intervals and an increase in cutaneous perfusion was seen in the two intervention groups (open or endovascular) of either +4.2% or +8.6%. The conservatively managed group was not assessed in this regard [6].
More recently IPC has again been recommended as an adjunct for this cohort of patients. Levin et al. [24] in 2020 produced an article about CLI in which they included a small cohort of studies that demonstrated that IPC can reduce amputation rates and help wound healing. They included it as an alternative therapy in this study.
This study is limited by the sample size. Ten devices were given to be used in this study and 10 patients were selected as it was deemed a pilot safety study. Because of this and the select nature of the patients, this study was a non-randomized trial. In future trials using this device, if a larger sample size is used and a randomized control trial is undertaken this may reduce any potential bias present in this study.
Finally, as well as being safe, this IPC has been shown to potentially decrease pain, improve wound healing and improve quality of life for wound patients. However, given the small size of the study, the statistical significance of this is in doubt. This was a pilot safety study and only 10 patients were selected. 10 Wound Express devices were provided by the device manufacturer without charge. The manufacturer was not involved in the protocol design, methodology, data collection and interpretation or the writing of this manuscript. Similar studies using this device have also demonstrated positive results. Naik et al. [25] showed a decrease in pain scores of 15 out of 18 patients with mixed etiology ulcers. Median pain scores went from 14 to 6.50 in an 8-week period wearing the device. They also noted that patient opiate requirement decreased. This may further add to the cost-effectiveness of the device.
It has also been shown to potentially improve patients’ quality of life with ulceration. Chronic lower extremity ulceration is a significant burden to many patients, and this device may be of benefit to them when other options are not available.
In conclusion, the Wound Express device is a safe device for use in arterial disease under close observation in patients with unreconstructible arterial disease. No adverse outcomes were seen related to the device. As mentioned, one patient died from an unrelated pathology and two withdrawals were deemed not related to the device. Potential improvements were seen in wound healing, pain scores and quality of life. Further larger and longer studies are necessary to assess fully the role this device may play in these difficult to manage wounds. A further area of study using the device may be to assess any improvement in collateralization using imaging, such as lower limb computerized tomography.
Notes
No conflicts of interest to declare, except as mentioned, Huntleigh Healthcare Ltd. (Cardiff, UK) provided 10 Wound Express devices for use at no charge. They were not involved in the study design or protocol, data collection or interpretation or drafting of the manuscript.
Acknowledgements
The authors would like to thank Huntleigh for allowing us to use the devices without charge and also to the patients for taking part in the study.
Supplementary material
Supplementary Fig. 1.
Wound Express device. (A) Control module is seen connected to thigh attachment. (B) It is placed on the thigh of the affected limb for 2 hours per day. Image courtesy of WoundExpress.com.
Supplemental data can be found at: https://doi.org/10.22467/jwmr.2022.02278.
jwmr-2022-02278-Supplementary-Fig-1.pdf