Utility of Indocyanine Green Fluorescence Imaging in Wound Assessment
Article information
Abstract
The use of indocyanine green fluorescence imaging has become popular in the field of reconstructive surgery as it allows surgeons to confirm flap vascularity and lymphatic flow. Based on its reliability in detecting tissue perfusion, it is now being used to evaluate the status of wounds which helps surgeons make accurate intraoperative and postoperative decisions. This article aims to provide an overview of indocyanine green fluorescence imaging and its current utility in assessment of various wounds.
Introduction
Fluorescence imaging has been widely used in biomedical research as it provides molecular information of cells by way of unique illumination. Its intuitive visualization allows high sensitivity and currently has become an essential immunochemical technique in laboratories. Among various fluorescent imaging agents, indocyanine green (ICG) is one of the most popular agents approved by the U.S. Food and Drug Administration (FDA) in 1959 [1] and its application has been widened from the laboratory to the clinical field. Clinically ICG was first used to evaluate liver function [2,3] and now is being used in such diverse diagnostic and surgical capacities such as fundus angiography, neurosurgery, cardiac surgery, and sentinel lymph node biopsy. The use of ICG fluorescence imaging has become especially popular in the field of reconstructive surgery as it allows surgeons to confirm flap vascularity and lymphatic flow. Based on the reliability of ICG fluorescence imaging when detecting tissue perfusion, it is now being utilized for evaluating the status of various wounds. In this article, we will review and discuss about the current status of ICG fluorescence imaging utilized in wound assessment. The patients provided written informed consent for the publication and use of their images.
ICG and fluorescence imaging devices
ICG is a chemical compound (C43H47N2NaO6S2) having a molecular weight of 751.4 Da [4]. It is a negatively charged ion that is soluble in water but not readily soluble in saline. Therefore, it is usually provided as a sterile lyophilized green powder and should be dissolved in sterile water. It binds tightly to plasma proteins in the vascular compartment without leakage and is metabolized in the liver with a half-life of 150 to 180 seconds [5]. The rapid excretion and low toxicity allow repeated application during surgery.
In ICG fluorescence imaging, radiation from the light source is filtered to remove fluorescent wavelengths. Blood and ICG agents in tissue exposed to this filtered light absorb excitation wavelengths and emit fluorescent bands which are received by the sensor [5]. ICG fluoresces around 780 nm with an emission peak towards longer wavelengths of 805 to 810 nm [6]. This allows up to 10 mm in tissue penetration depth, capturing the vasculature in the deep dermal plexus and subcutaneous fat without the risk of tissue damage [7]. Light sources most frequently used for fluorescence imaging are light emitting diodes (LED), diode lasers, and halogen lights. The first fluorescence imaging device for surgery was invented in 2005 by Novadaq (SPY SP2000). Microscopes with fluorescence imaging modes were also introduced by Carl Zeiss and Leica which allowed neurosurgeons to utilize fluorescence imaging in central nervous system surgery. Currently, portable handheld fluorescence imaging devices are frequently used by plastic and reconstructive surgeons to detect vascularity or lymphatics not only intraoperatively but also pre- and postoperatively at the ward or outpatient clinic. Highlighting the evolution of simpler portable devices, Mito et al. [8] demonstrated the use of smartphones as an imaging device.
ICG fluorescence imaging in wound assessment
Acute wound assessment
Burn wounds
Historically, the first use of ICG fluorescence imaging for wound assessment was to estimate burn depth in an animal study [9]. It was then applied to human burn depth estimation in 1995 [10]. Clinically, estimating burn depth to distinguish between superficial partial-thickness and deep partial-thickness burns is critical since this differentiates whether a patient can be healed by dressings for secondary intention or may need early debridement followed by a skin graft. Burn depth is generally assessed by the surgeon through physical examination, including looking for the presence of blisters, the appearance of the dermis, and the color of tissue. This differentiation can take a couple of days or weeks to wait and see the progress of the burn wound for a confident diagnosis; nonetheless the accuracy of such assessments has been shown to be only 60% to 75% [11]. In a prospective, multicentered, triple-blinded, experimental study by Wongkietkachorn et al. [12] on a total of thirty indeterminate burn wounds, the accuracy of ICG angiography was 100% compared with 50% for clinical assessment. More accurate burn depth estimation lead to more precise debridement that ultimately is related to better skin graft survival [13].
Traumatic wounds
ICG fluorescence imaging can be applied in acute traumatic wounds to assess tissue viability which in many instances is difficult to determine clinically until demarcation which can take several days to weeks. Degloving injuries are a challenging example for reconstructive surgeons to manage as they usually need some time until the soft tissue is demarcated. Appropriate debridement to preserve as much viable tissue as possible is the most critical procedure for successful reconstruction and long-term function. A recent study used ICG fluorescence imaging to evaluate degloved tissue perfusion of the plantar foot area in the early post-traumatic period which was highly useful for debridement and also for reducing the risk of infection [14]. ICG angiography was also utilized to assess tissue viability in an avulsed scalp, guiding the clinical decision to perform microanastomosis (Fig. 1) [15].
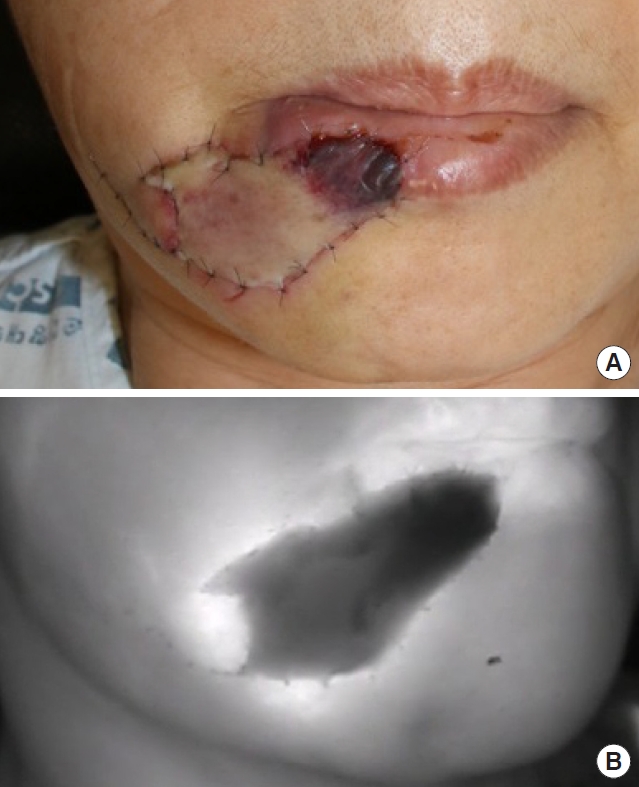
Indocyanine green (ICG) fluorescence imaging for a degloved lower lip. (A) Clinical photograph on postoperative day 3 displaying a partially demarcated flap. (B) ICG fluorescence imaging demonstrated flap non-viability, enabling the surgeon to decide on early debridement and revision surgery.
High-energy open fractures combined with surrounding soft tissue injuries can be another indication to apply ICG fluorescence imaging for locating and debriding all devitalized or poorly perfused bone and tissue [16-18]. ICG fluorescence imaging may also help minimize the number of procedures since this type of injury usually requires multiple sessions of surgical debridement and a long length of hospital stay. In complex vascular trauma cases, ICG angiography can also aid decision-making for major considerations such as choosing between attempting revascularization or proceeding with amputation [19]. ICG fluorescence imaging has furthermore proven its efficacy in war-related trauma where all kinds of acute traumatic wounds are encountered [20].
Operative wound assessment
Breast reconstruction
Currently, ICG fluorescence imaging is most frequently utilized in elective breast surgery including skin- or nipple-sparing mastectomy and locoregional or free flap reconstruction (Fig. 2). Skin or nipple areolar complex necrosis after mastectomy is a common complication which may result in an aesthetically disfigured breast. Intraoperative assessment of mastectomy skin flap viability is a crucial step to minimize this potential disfiguration by enabling surgeons to predict in advance skin flap survival and necrosis and thus safely reconstruct the breast. Numerous studies have reported on the evaluation of mastectomy flap viability with ICG angiography in both immediate and delayed breast reconstruction; their results demonstrated that ICG angiography reduced postoperative complications compared to clinical evaluation [21-25]. In free flap breast reconstruction cases, ICG angiography can help surgeons predict Hartrampf’s zones of perfusion and decide whether to super- or turbo-charge flaps to prevent fat necrosis (Fig. 3) [26-28].
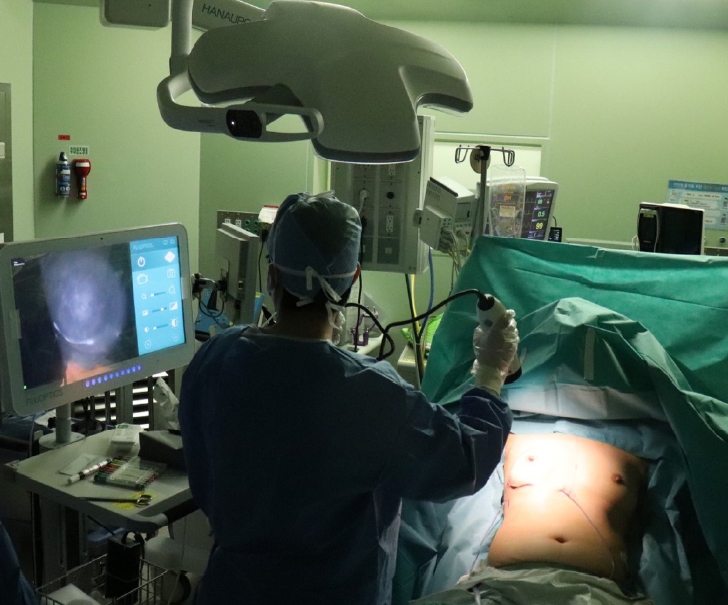
Demonstrative photograph of indocyanine green (ICG) angiography. ICG angiography performed during breast reconstruction immediately after nipple-sparing mastectomy.
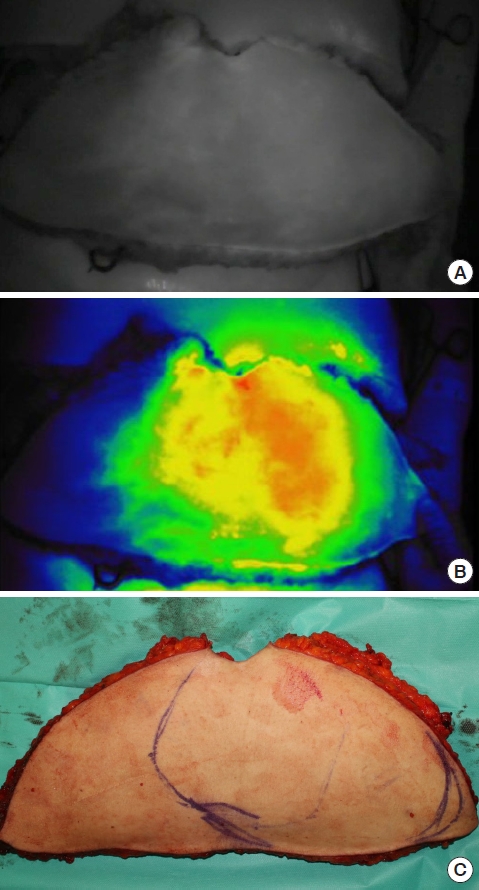
Indocyanine green (ICG) fluorescence imaging for free flap surgery. (A) ICG fluorescence imaging of a deep inferior epigastric artery perforator flap showing the area of perfusion. (B) Red color represents well-perfused areas while blue color shows poorly perfused areas of the flap. (C) Designing areas of the flap to be sacrificed according to ICG fluorescence imaging.
Flap reconstruction
Similar to free flap breast reconstruction, ICG angiography is utilized in various other types of free flap surgery including lower extremity or head and neck reconstruction to minimize postoperative complications from flap necrosis [29-31]. Even partial flap necrosis can lead to a chronic fistula, especially in coverage of orofacial defects; therefore assessing the reliably perfused area of flaps is crucial to prevent this complication. ICG angiography is utilized not only in free flap reconstruction but also in locoregional flap reconstruction during flap design and inset to improve flap survival [32-34]. In addition, it is further used to assess flap vascularity prior to flap division in distant flaps such as the cross-leg flap and paramedian forehead flap [35,36].
Postoperative flap monitoring can also be done by ICG angiography. Assessment of flap survival is still done clinically in most institutions by monitoring the flap capillary refill time, color and warmth, or bleeding, which are all subjective measures. Some more objective methods such as implantable Doppler probes and color duplex ultrasound have difficulties in interpretation and require mastering a steep technical learning curve. However, ICG angiography visually presents flap viability in black and white and allows direct visualization and immediate decision-making for salvage procedures [37,38].
Chronic wound assessment
Although ICG fluorescence imaging has been mainly used for assessing either acute or surgical wounds, research has also been carried out on its application to chronic wounds. Most studies demonstrated the use of ICG angiography in critical limb ischemia patients to quantify perfusion and prognosticate wound healing [39,40]. ICG angiography has also been used to evaluate the outcomes of revascularization procedures and bypass surgery for peripheral arterial disease [41,42]. When performing amputation in lower limb ischemia patients, ICG angiography can be used to predict and detect tissue perfusion in amputation stumps which have a high risk of necrosis, therefore preventing repetitive revision surgery which otherwise would be frequently necessary (Fig. 4) [43,44]. Some recent studies report the effects of hyperbaric oxygen therapy on chronic wounds such as diabetic foot ulcers and radiation necroses as evaluated by ICG angiography [45,46].
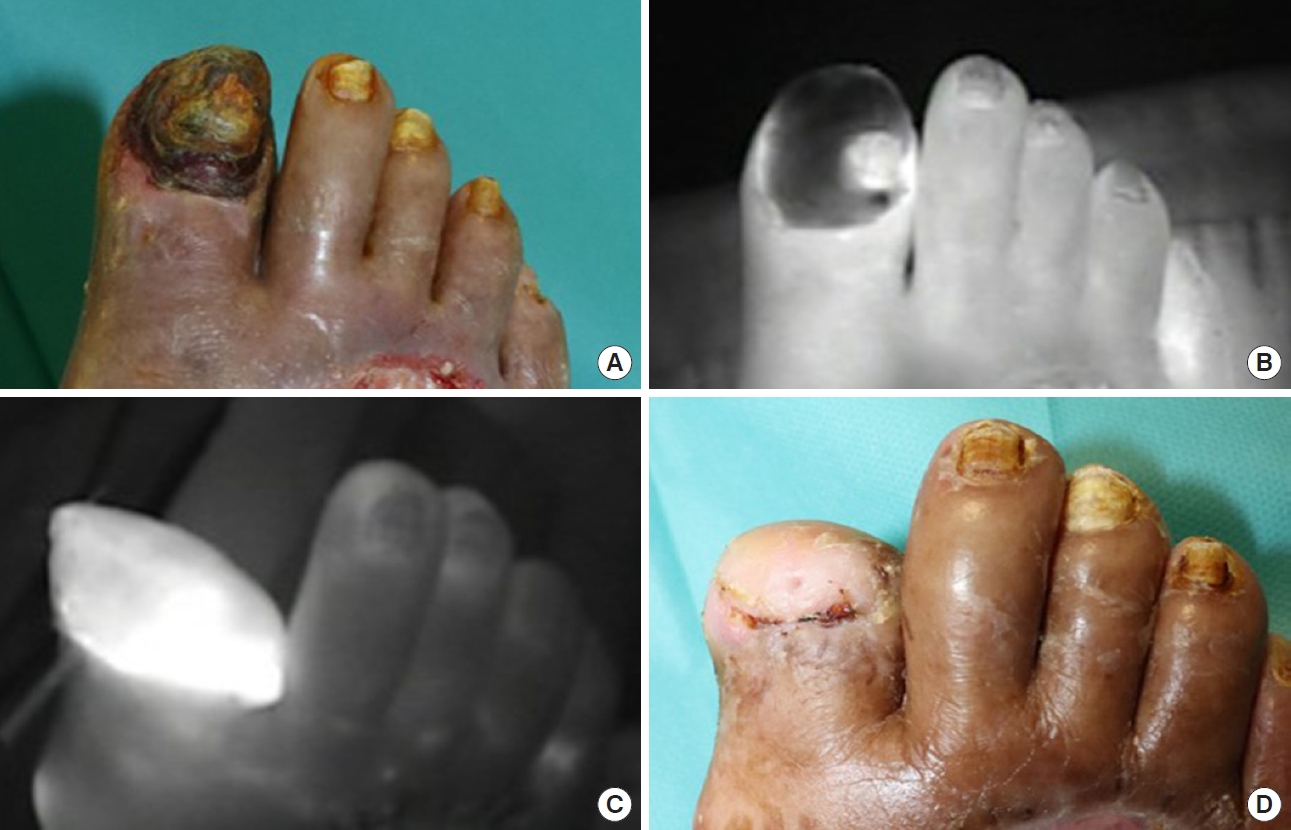
Indocyanine green (ICG) fluorescence imaging for toe amputation. The patient suffered from severe peripheral arterial disease. (A) Preoperative clinical photograph and (B) ICG fluorescence imaging of necrotized right great toe. (C) Immediate postoperative ICG fluorescence imaging after amputation and fillet flap coverage. (D) Clinical photograph at postoperative day 14.
Limitations of ICG fluorescence imaging
Although ICG fluorescence imaging has recently gained pop ularity especially among plastic and reconstructive surgeons, there are some concerns and limitations that need to be acknowledged. First, the appropriate dosage of ICG used for wound assessment has not been established. Doses used in various studies range from 7.5 to 25 mg [21,24,47]. ICG is generally packed as a powder to be mixed with diluents and injected as an intravenous bolus. ICG has been administered safely to children intravenously in doses up to 0.5 mg/kg to assess and reduce the risk of postoperative flap necrosis in various sites of acute pediatric trauma [48]. More exact safe concentrations and doses of ICG should be established to avoid allergic reactions. Second, the timing of assessment after ICG injection also varies among studies. Many studies describe image recording for a period of 2 minutes immediately after injection while some studies started to record after a latency period (1–3 minutes). Furthermore, when repeating ICG evaluation in the same patient, uncleared dye from previous injections may cause confusion. Although the half-life of ICG is known to be very short, it has been reported to increase to up to 24 hours if ICG binds to a plasma protein HDL [49]. Most importantly, false-positive and false-negative cases should be resolved to attain higher sensitivity and specificity. More precise modalities to analyze quantitative data must be employed in fluorescence imaging devices to exactly distinguish viable tissue boundaries.
Conclusion
ICG fluorescence imaging has become an important surgical tool for reconstructive surgeons in acute, surgical, and chronic wound assessment. It may help surgeons make more accurate decisions intraoperatively and postoperatively, leading to superior functional and aesthetic outcome.
Notes
No potential conflict of interest relevant to this article was reported.
