Effect of Low-Level Laser Therapy on Proliferation and Collagen Synthesis of Human Fibroblasts in Vitro
Article information
Abstract
Background
Low-level laser therapy (LLLT) is a nonthermal technology that can be used to modulate cellular activity through light irradiation at specific pulse sequences. in vitro and in vivo studies have been performed previously to determine the effect of LLLT on wound healing. However the results were inconsistent. Final purpose of our project is to determine effect of LLLT on diabetic wound healing and this pilot study was designed to confirm effect of LLLT on activity of healthy human fibroblasts. In particular, we focused on cell proliferation and collagen synthesis, which are main contributing factors in would healing.
Methods
Healthy human fibroblasts obtained from cryopreserved cells (n=10) were irradiated at wavelength of 635 nm (RED), 830 nm (IR), and 635 nm+830 nm (FX) with the same fluence of 60 J/cm2 , after seeding into 96-well plates. A group in which no laser exposure was applied was assigned as control. Fibroblast proliferation was examined by EZ-Cytox enhanced cell viability assay and immunohistochemistry (IHC). Collagen synthesis was measured by IHC. IHC pictures were analyzed to identify the intensity values of collagen type I as quantity results.
Results
Irradiation at FX and IR groups showed a significant increase in fibroblast proliferation and collagen synthesis compared to control and RED groups. There was no significant difference in fibroblast proliferation and collagen synthesis between FX group and IR group.
Conclusion
Healthy human fibroblasts showed better cell proliferation and collagen synthesis when they were irradiated at wavelength of 635 nm+830 nm or 830 nm.
Introduction
The prevalence of chronic wounds is increasing dramatically, as the populations of industrialized countries age and become more sedentary. Chronic wounds that respond poorly to conventional treatment, making them very difficult to manage [1]. It is well-known that fibroblasts provide desired growth factors and other substances to accelerate wound healing. However, fibroblasts from chronic wounds such as diabetic ulcers have been commonly demonstrated a lower rate of proliferation when compared with healthy ones [2]. Therefore, it is significant to increase fibroblast activity for accelerating wound healing. The care of chronic wounds has become its own specialty, with providers often using advanced therapies, including growth factors, extracellular matrices (ECMs), engineered skin, and negative pressure wound therapy (NPWT) [3].
Low-level laser therapy (LLLT) is a nonthermal technology that can be used to modulate cellular activity through light irradiation at specific pulse sequences [4]. in vitro and in vivo studies have been performed previously to determine the effect of LLLT on wound healing. However the results were inconsistent. Using a variety of red, blue, yellow, and infrared light wavelength, it has been reported that LLLT can accelerate cutaneous wound healing [5]. Clinical benefits of using light irradiation through LLLT on skin wounds are also reported in the literature [6]. Furthermore, it has been reported that LLLT enhances cell proliferation of several cell types including fibroblasts, endothelial cells [7]. However, some studies have suggested that LLLT might have no effect on fibroblast proliferation and activity [8,9]. Inhibitory effect on fibroblast proliferation and activity using red light or infrared light were also reported in literature [10,11]. Many studies have demonstrated that the effect of light irradiation on human skin fibroblasts is dose and/or wavelength dependent [7-11].
Final purpose of our project is to determine effect of LLLT on diabetic wound healing, which is one of representing chronic wounds. Moreover, this pilot study was designed to confirm effect of LLLT on activity of healthy human fibroblasts. In particular, we focused on cell proliferation and collagen synthesis, which are main contributing factors in would healing.
Methods
Human dermal fibroblast culture
Dermal fibroblasts were obtained from cryopreserved cells derived from the dermis of 10 healthy adults who had provided informed consent for their cells to be used for research purposes. Fibroblasts were cultured with Dulbecco’s modified Eagle medium/Ham’s F-12 nutrient (DMEM/F-12; Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco) and 25 mg/mL of gentamycin) in 100-mm tissue culture dishes (Corning Life Sciences, Corning, NY, USA) at 37°C. All cultures in this study were performed at 37°C in a 5% CO2–95% air atmosphere. Once the culture reached approximately 80% confluent, fibroblasts were dissociated by trypsinization, diluted 2.7-fold in phosphate-buffered saline without Mg2+ and Ca2+ (PBS; Gibco), and collected by centrifugation at 450 g for 17 minutes. Cell density was determined by trypan blue dye exclusion test.
To determine the effect of lasers on cell proliferation, 3× 103 cells in 100 μL DMEM/F-12 with 5% FBS were seeded into each well of sterile 96-well culture plates (Fisher Scientific, Pittsburgh, PA, USA). For Immunohistochemistry (IHC) study to evaluate cell proliferation and collagen synthesis, 5×103 cells in 200 μL DMEM/F-12 with 5% FBS were seeded into each well of sterile 8 well cell culture slides (SPL Life Science, Pocheon, Korea). These 96-well plates and 8-well slides were then incubated at 37°C overnight to allow human fibroblasts to attach.
Laser irradiation
Human fibroblasts were irradiated using Light-emitting diode (Smartlux, MEDMIX, Incheon, Korea) in a light room. Three different wavelengths were treated; red light (RED; 635±6 nm), infrared light (IR; 830±5 nm), and a dual-wavelength output light (FX; RED+IR). Cells were irradiated with continuous wave at a fluence of 60 J/cm2 on day 1 and day 3 (48 hours interval between irradiations). The distance from light source to fibroblasts was 15 cm [12]. Human fibroblasts without any laser exposure were used as controls. Control group plates were taken out of the incubator at the same time and placed in the same ambient environment as their matched treatment plates. On day 5 (48 hours after laser irradiation), cell proliferation and collagen synthesis were measured.
Cell proliferation assay
On day 5, 96-well plates were subjected to cell proliferation analysis using EZ-Cytox Enhanced Cell Viability Assay Kit (DoGen, DAEILLAB, Seoul, Korea). EZ-Cytox assay measures cell mitochondrial activity based on the conversion of water-soluble tetrazolium salt WST-1 (4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1, 3-benzene disulfonate) to insoluble formazan. Briefly, cells were incubated with WST-1 reagent for 3 hours. Absorbance was then measured at wavelength of 450 nm using a 96-well plate reader with reference wavelength of 600 nm.
In addition, 8-well slides were subjected to cell proliferation analysis using IHC staining. Each group of human fibroblasts was washed twice with ice-cold PBS, fixed with 4% paraformaldehyde (pH 7.4) in 8-well slides for 30 minutes on ice. Removed the fixative and wash 3 times for 5 minutes each with PBS. Then incubated fibroblasts with 0.5% Triton X-100 in PBS for 10 minutes at room temperature to permeabilize the membranes, and rinsed three times with PBS. Endogenous peroxidase was then blocked by incubating with 3% H2O2 in PBS at room temperature for 30 minutes. After rinse with PBS 3 times, the 8-well slides were then incubated with primary antibody at 4°C overnight. After washing with PBS 3 times, the slides were incubated with corresponding fluorescent dye-conjugated secondary antibody at 37°C for 1 hour (protected from light). After fixation, sections were rinsed 3 times with buffer and mounted in 25 μL Vector shield with DAPI (4´-6-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA, USA) for staining nuclei.
Collagen synthesis assay
Collagen type I synthesis assay was performed using IHC staining. The detailed procedure has been described in cell proliferation subsection. These 8-well slides were incubated with primary antibody (anti-collagen type 1; Abcam, Cambridge, MA, USA) and secondary antibody (anti-rabbit-FITC; 1:200, Abcam) for staining collagen type I.
Image acquisition of IHC
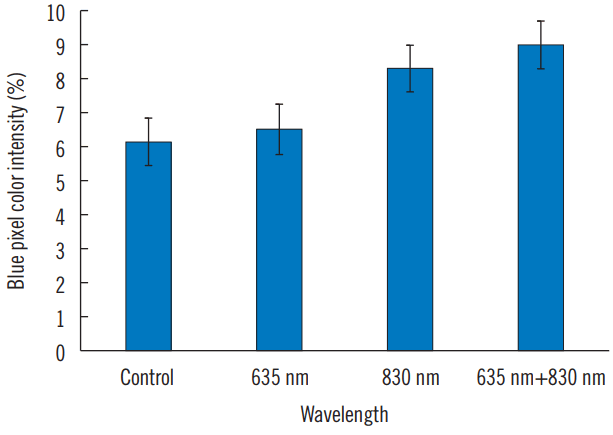
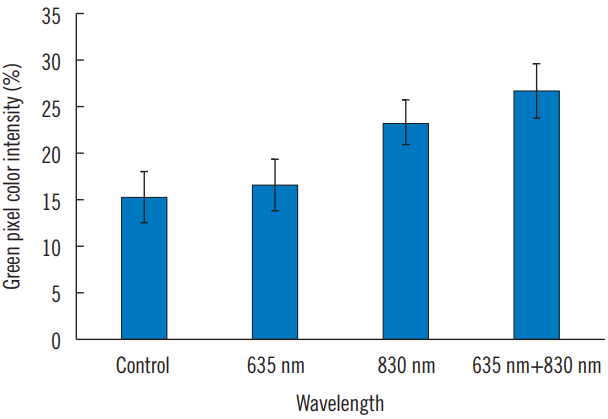
All IHC pictures were taken at the exact center of 8-well slides (200×magnification). Images were analyzed to identify the intensity values of blue color pixel and green color pixel as quantity results, for the reason that nuclei stained by DAPI showed blue color, while collagen type I stained by FITC showed green color in IHC images. Whole slide image analysis was performed using positive pixel count using Adobe Photoshop CS6 (Adobe Systems, San José, CA, USA).
Statistical analysis
All experiments were performed in triplicates and average value was used as each data. Results are expressed as means±standard deviations. Statistical comparisons were performed with Wilcoxon signed ranks test and paired t-test. Statistical significance was considered at P<0.05. Data were analyzed using Statistical Product and Service Solutions version 20 (SPSS; Systat Software, IBM, Armonk, NY, USA).
Results
Cell proliferation
In cell proliferation analysis using EZ-Cytox Kit, FX group (635 nm+830 nm) demonstrated highest cell number, followed by IR and RED groups. Cell proliferation in the FX and IR groups showed statistically significant differences compared with RED and control group. However, there was no statistically significant difference between FX and IR groups (Fig. 1 and Table 1). Results of IHC study are shown in Figs. 2, 3, and Table 2 which were consistent with results of EZ-Cytox analysis.
Collagen synthesis assays
Collagen synthesis was increased in all irradiated groups compared to that in the control group. However, statistically significant differences were detected in IR group and FX group (Figs. 4, 5, and Table 3).
Discussion
According to previous research, LLLT has effect on energy and absorption levels of cells relevant to their respiratory chains [13]. This indicates that LLLT can stimulate components of the so-called antenna pigments of the respiratory chain of cells by using electromagnetic energy, therefore sensitizing cells by increasing mitochondrial ATP production [14]. Electromagnetic radiation in the form of light can vitalize macromolecules depending on wavelength. It can initiate conformation changes in proteins and transfer energy to electrons [15]. Absorption of a specific wavelength of light can affect functioning photoreceptor (photoacceptor) molecule in the metabolic pathway involving redox chains [16]. It is known that an action spectrum follows the absorption spectrum of the photoacceptor molecule within certain limits [13,16]. For these reason, wavelength can play a fatal role in LLLT. As mentioned earlier, the preferred wavelength to stimulate fibroblast proliferation at visible red light or invisible infrared light remain controversial.
Another optimal parameter for LLLT is fluence. Exposure to lower-fluence laser light (from 0.09 to 20 J/cm2) [17] and medium-fluence laser light (20–80 J/cm2) [11,13] tends to accelerate cell growth and wound-healing, whereas higher-fluence light (more than 80 J/cm2) [18] negates the beneficial effect of laser exposure. However, no significant difference or inhibited effect in fibroblast proliferation has also been found using lower-fluence laser light in several studies [8,10]. Therefore, lower-fluence laser light remaining controversial also would not be an option for our study. A similar conclusion has also been found by Luciana Almeida-Lopes [19]. As a result, the better choice of fluence for our study was to use one of medium-fluence laser light [4,12]. The fluence at 60 J/cm2 was used in our study. However, a dose-dependent study is needed in the future to definitely determine its effect on cell proliferation and collagen synthesis of fibroblasts.
Our findings indicated that LLLT at wavelength of 830 nm or 635 nm+830 nm with fluence of 60 J/cm2 could modulate fibroblast proliferation and collagen synthesis, with cells irradiated at 635 nm+830 nm possibly giving better results. It is difficult to compare our results with observations of other studies in the literature, because most studies have reported only one or two different single wavelength and treatment design varies depending on individual studies [10,11]. However, some studies have been performed in a similar manner to our study [12,20,21]. Bogdan Crisan has compared near-infrared laser light at wavelength of 830 nm, 980 nm, and 2,940 nm. Whereas, these three wavelength are actually is in the same spectrum. Among these three wavelength, irradiation at 830 nm showed the most notable bio-stimulation effect on human skin fibroblasts [21]. For comparison between two kinds of spectrum (visible red light and infrared light), some studies have demonstrated that infrared low level lasers are preferred over visible red light lasers in stimulating cell proliferation or collagen synthesis [12,19]. These results are similar to results of the present study. It has been reported that 780 nm laser light can up-regulate the production of basic fibroblastic growth factor (bFGF) more than 660 nm visible red laser [12]. Diabetic fibroblasts irradiated at 830 nm have shown more bFGF release compared to those irradiated at 632.8 nm [20].
Previous studies have demonstrated action mechanisms of visible to near-infrared radiation on cells. It has been suggested that we should pay an attention to energy values relevant to the respiratory chain of cells. The effect of electromagnetic wavelength on cellular energy transfer has become evident [16]. Tiina Karu reported that absorbing molecule can transfer energy to another molecule and that the activated molecule can then cause chemical reactions in surrounding tissue as the basis for LLLT effect [13]. Moreover, cytochromes c oxidase (red and near-infrared light region) is one of the primary photoacceptors reported in the literature. Cofactors for cytochromes c oxidase and cytochromes b, c1, and c are porphyrins while cofactors for complex I (an enzyme of the respiratory chains) are flavins [22]. These changes in mitochondrial respiratory chain can mediate the activation or suppression of signal molecules in the cytoplasm, causing subsequent changes of downstream cascades and leading to the synthesis of DNA, RNA, proteins, and enzymes in the nucleus, cytoplasm, or plasma membrane. Finally, these changes will result in photobiological effects of cells such as cell proliferation and differentiation [23, 24]. Therefore, we speculated that dual-wavelength (635 nm+830 nm) could have better effects on fibroblast proliferation and collagen synthesis through both red region and near-infrared region action spectrum by increasing the range of photoacceptor molecule. However, according the present study, we can’t demonstrate that there are significant difference in cellular response of human fibroblasts between 830 nm and 635 nm+830 nm, may be due to the less amount of case. A further study is needed in the future to confirm it. The results of the present study may suggest that dual-wavelength light (635 nm+830 nm) could be an optimal choice for LLLT to improve the wound-healing. Our results might help clinicians in the choice of wavelength appropriate for LLLT.
As a conclusion, our study demonstrated that dual-wavelength light (635 nm+830 nm) or infrared light (830 nm) has stimulating effect on proliferation and collagen synthesis of human fibroblasts in vitro. However, visible red light (635 nm) does not.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This article was presented as a oral presentation at the Internarional 75th Congress of the Korean Society of Plastic and Reconstructive Surgeons on Nov 10–12, 2017.