Introduction
Pleomorphic dermal sarcoma (PDS), which arises from the skin, is a new type of soft tissue sarcoma that is regarded as both a cutaneous form of undifferentiated pleomorphic sarcoma and an aggressive version of atypical fibroxanthoma [1]. Owing to its rarity, the overall prevalence of PDS has not yet been reported. PDS often appears to be aggressive, with a characteristic presentation of nodular or polypoid tumors with rapid growth on areas exposed to ultraviolet (UV) light, such as the scalp or face [2,3].
Malignant transformation of chronic burn scars is a well-known phenomenon, but most cases are of ectodermal origin, such as squamous cell carcinoma (SCC) or basal cell carcinoma. Sarcomas arising from burn scars have rarely been described [4,5], and we are not aware of any reported cases of PDS developed upon burn scars.
Here, we report a unique case of PDS originating from a burn scar on an UV-independent location. In addition, we discuss the diagnosis and management of this rare mesenchymal malignancy and highlight the pathophysiology of burn scar neoplasms. This case may help clinicians to promptly diagnose and treat this rare, aggressive soft tissue tumor and to be more aware of burn scar neoplasms. The report was approved by the Institutional Review Board of SMG-SNU Boramae Medical Center (IRB No. 30-2022-14). The patient provided written informed consent for the publication and use of his images.
Case
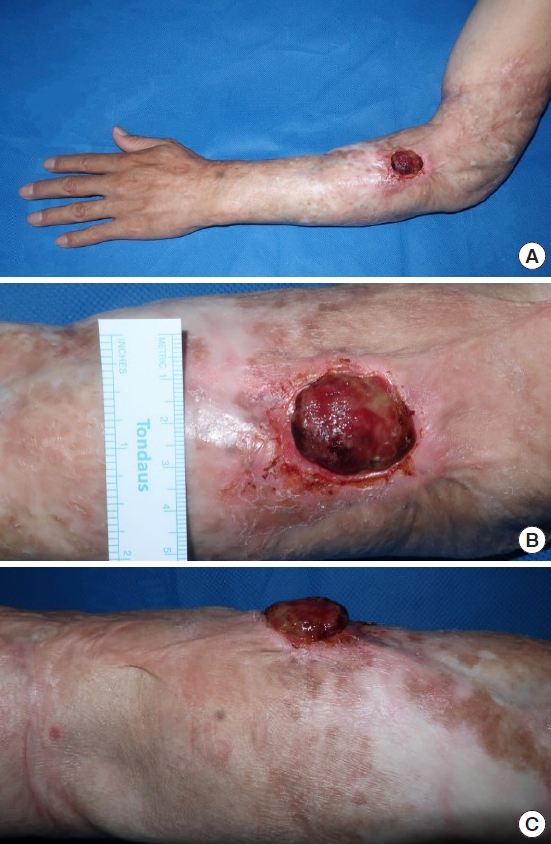
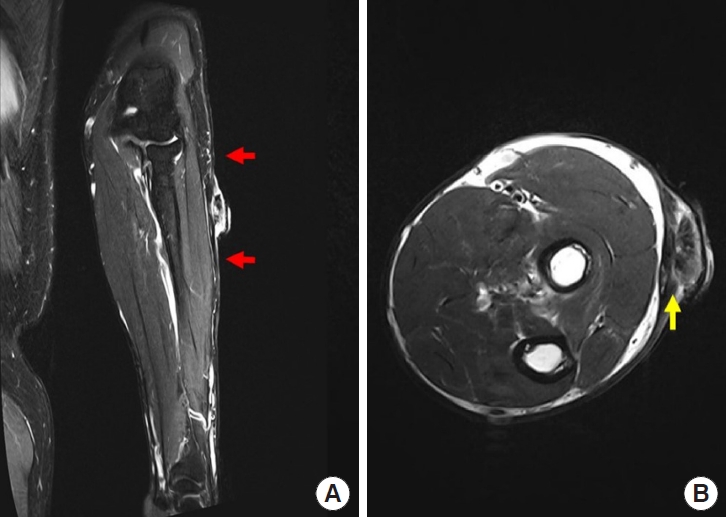
A 52-year-old man presented with a protruding ulcerative mass on his left arm 3 months after its appearance. He had sustained a scalding burn injury on his left arm 40 years earlier and had not sought professional medical care at that time, leaving a burn scar on his entire left forearm and distal part of the left upper arm, with mild elbow joint contracture. The patient tended to cover his arm with a long-sleeved shirt because he did not want others to be aware of his scar, which minimized sunlight exposure. Three months prior to presentation, incidental scratching resulted in a mild abrasion on the left forearm scar. Crusting and bleeding repetitively appeared on the abrasion site, resulting in progression to an exophytic ulcerated mass (Fig. 1). The protruding eczematous mass, approximately 3.0×2.9×1.2 cm in size, was located on the dorsal side of the left forearm. Punch biopsy of the mass suggested the possibility of PDS. Forearm magnetic resonance imaging revealed diffuse dermal thickening and enhancement adjacent to the protruding mass, with suspected subcutaneous layer involvement (Fig. 2). Upper extremity computed tomography (CT) revealed no major vascular structures near the lesion. Chest CT and whole-body positron emission tomography-CT scans for additional staging revealed no evidence of metastatic axillary lymph nodes or distant metastases. In addition, upper extremity lymphoscintigraphy confirmed decreased lymphatic flow in the left upper extremity (Fig. 3).
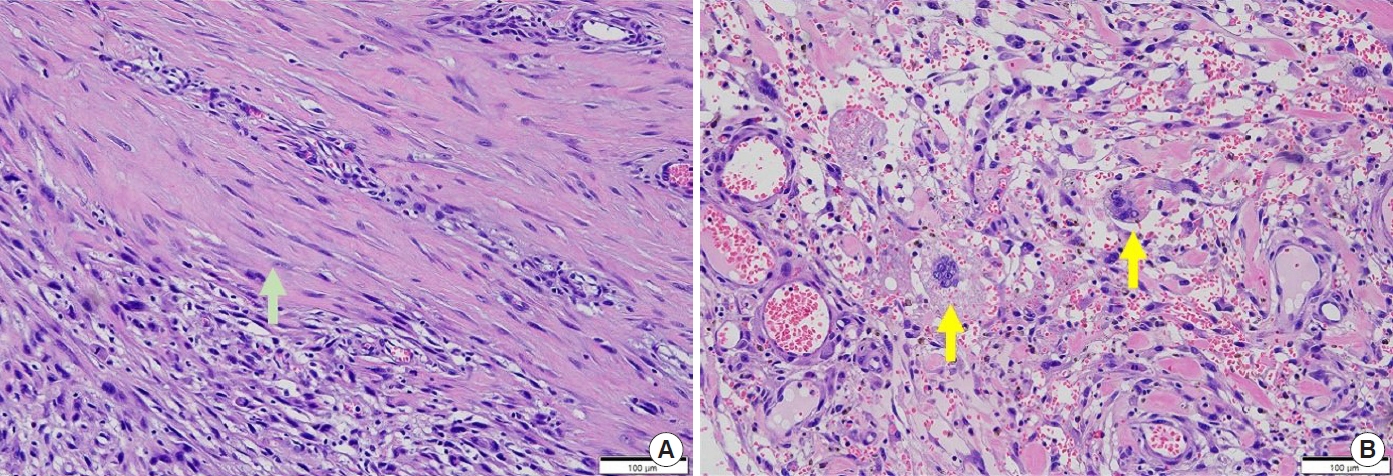
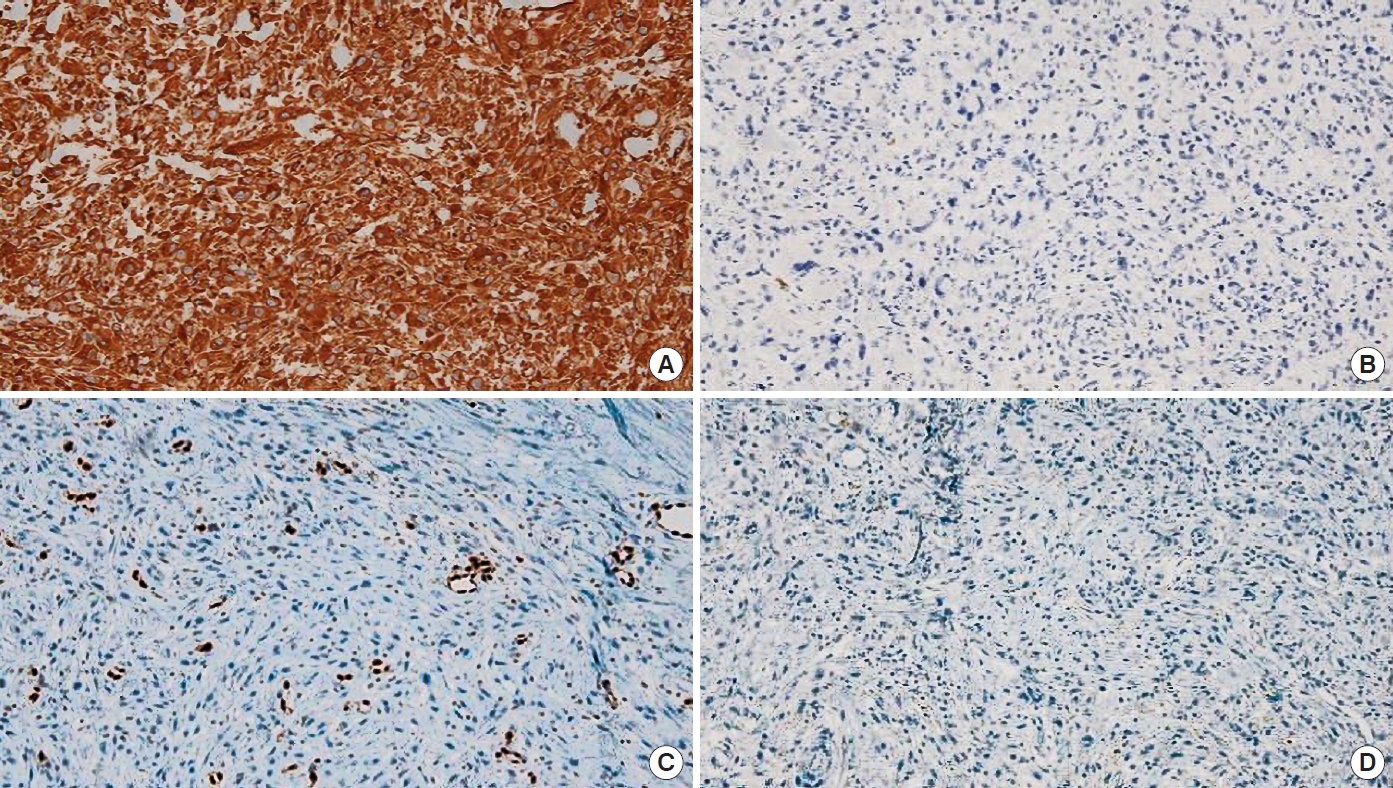
Wide excision of the mass was performed, with a 2-cm margin, including the entire subcutaneous layer but preserving the fascia. The post-excision skin defect was an estimated size of 10.5×5.5 cm, and a split-thickness skin graft from the left anterior thigh was used to cover the defect with concomitant one-stage acellular dermal matrix (AlloDerm, LifeCell Corp.) application (Fig. 4). Pathologic examination revealed a tumor mass of 3.2×2.5×0.8 cm in size with a clear resection margin, diagnosed as a high grade (Trojani grade III) pleomorphic and spindle cell sarcoma, with a mitotic count of 11 per 10 high power fields, and necrosis accounting for 20% of the tumor area. No angiolymphatic invasion was observed (Fig. 5). Other skin cancers were excluded after immunohistochemistry (ERG [avian v-ets erythroblastosis virus E26 oncogene homolog] focal: weakly positive, p63: negative, cytokeratin: some positive cells, vimentin: positive, S100 protein: negative, CD68: positive, and leukocyte common antigen: negative in atypical cells), resulting in a final diagnosis of PDS (Fig. 6). The patient exhibited total skin graft integration and no evidence of local recurrence at the 1-year follow-up after the operation. He will be routinely followed-up for local recurrence and systemic relapse every 6 months for at least 5 years.
Discussion
As the diagnosis of PDS is based on its histopathologic characteristics, a routine immunohistochemical work-up is a prerequisite to exclude other skin cancers. Histologically, PDS is based on the dermis without a connection to the epidermis or grenz zone and is composed of pleomorphic epithelioid, spindled, and multinucleated giant tumor cells arranged in sheets and fascicles. It is accompanied by deep subcutaneous tissue invasion, tumor necrosis, and lymphovascular invasion. Upon immunohistochemical examination, PDS exhibits negative test results for multiple combinations of cytokeratin panels, which are positive in the case of SCC. PDS also exhibits low expression levels of melanocytic markers, such as S100, desmin and HMB-45, and of vascular stains, such as CD31, ERG, and CD34. The above histopathologic results are diagnostic of PDS [2,3,6-9].
Given the rarity of this type of tumor, there are no standard ized guidelines for the diagnosis and management of PDS; therefore, PDS is staged using the TNM classification system for soft tissue sarcomas and managed in the same way as other aggressive malignant sarcomas. Considering the study by Soleymani et al. [10], in which the local recurrence rate was approximately 20% for cases of incompletely resected PDS, complete surgical excision with at least a 1-cm resection margin is vital. Tardio et al. [2] and Miller et al. [3] reported a metastatic rate of 10% to 20%, mainly involving the lung, skin, or lymph nodes.
Although not fully understood, we expect that the pathophysiologic mechanism of burn scar-originating skin cancer is distinguishable from that of conventional skin cancers, as burn injuries lead to structural and functional changes in the skin. Among the various theories regarding development of burn scar neoplasms, our case is best explained by the theories of an immune-privileged environment, chronic irritation, and chronic toxin exposure.
A burn scar is a typical example of an immunocompromised district [11]. The dermis is an essential layer of skin that regulates the balance of immunocompetent cells, blood and lymphatic circulation, cytokines, peripheral nerve fibers, and neuropeptides. Therefore, deep burns, which are characterized by the replacement of the normal papillary and reticular dermis by inelastic fibrous bundles, result in destabilization of the immune microenvironment. Lymphoscintigraphy of our patient revealed decreased lymphatic flow in the injured upper extremity. Given that our patient’s burn scar was dry and hairless, we speculate that he had sustained a deep second-degree burn at the time of the insult, resulting in deformation of the dermis [11,12]. T-cell exhaustion and the formation of an M2-macrophage-rich microenvironment have been suggested as immune-evasion mechanisms of PDS development [13].
In addition, the chronic irritation theory posits that cellular hyperplasia and increased mitotic activity during repeated damage and healing cycles contribute to malignant transformation [14]. This may help explain why sarcomas occur much less frequently than epidermal malignancies such as SCC, as deep tissues are probably less vulnerable to stimuli than the epithelial layer during physical irritation. Our patient caused irritation of the lesion by repeatedly removing the crusts from the burn scar as soon as they formed during the 3-month latent period.
Finally, the toxin theory posits that toxins released over a long period by damaged tissues lead to cellular mutations. This case of PDS originating from a burn scar was likely affected by endogenous toxins. In burn scars with eczematous changes, as in our case, nitric oxide and other oxygen radicals are produced by inflammatory cells and cause endogenous insults. One of the most common carcinogenic gene mutations caused by endogenous insults is the TP53 mutation, which is observed in almost all cases of PDS [15]; however, it was not assessed in our case.
Considering the mechanisms described above, clinicians should keep in mind that burn scars can facilitate tumor progression to PDS, a disease with recurrence and metastasis rates as high as those of invasive SCC. SCC is the most frequent diagnosis in burn scar neoplasms, and invasive SCC is difficult to distinguish clinically from PDS. Both are highly related to UV-induced pathogenesis and are associated with a history of repeated itching, bleeding, and crusting. However, PDS does not exhibit scales, which are common in SCC. In addition, exophytic growth, a characteristic feature of PDS, is less common in the case of SCC. Therefore, when encountering a chronic, eczematous wound on a burn scar, with rapid exophytic growth, clinicians should consider the possibility of PDS and urgently perform aggressive management, including a metastasis work-up.