Jung, Choi, Na, and Wee: Coverage of Midline Back Wounds with Perforator-Based Pedicled Fasciocutaneous Flaps
Abstract
Background
The number of patients with midline back wounds is increasing. Midline back wounds may cause skin and soft tissue defects, resulting in cavity formation and bone or orthopedic metallic hardware exposure. These complexities complicate coverage of the wounds. This study aimed to determine whether a perforator-based pedicled (island) flap was useful for the coverage of midline back wounds.
Methods
After debridement of the wound, flaps were designed, dissected and inset into the midline wounds. The donor site was closed primarily. If the wound was too large to be covered with a unilateral flap, another flap at the contralateral site was used. Immediate and early postoperative position changes were made.
Results
Between May 2011 and November 2019, 13 patients with skin and soft tissue defects of the midline back underwent coverage with perforator-based pedicled fasciocutaneous flaps. All flaps survived. Though flap tip necrosis and/or wound dehiscence occurred in five patients, total flap necrosis was not observed. Immediate postoperative position change was possible, allowing the patient to avoid flap ischemia.
Conclusion
The perforator-based pedicled fasciocutaneous flap can be a useful option for covering midline back wounds with skin and soft tissue defects.
Key Words: Perforator flap; Pedicled flap; Surgical wound dehiscence; Surgical wound infection
Introduction
Midline back wounds can be caused by various reasons including orthopedic spinal surgery, pressure sores, trauma, infected epidermal or pilonidal cysts, or burns. More postoperative wound complications are observed as the number of spine surgeries increase [ 1], ultimately increasing surgeries to cover midline wounds. Many methods have been used to cover midline back wounds, including primary closure, skin grafts, and random local flaps. However, prominent spinous processes sometimes complicate covering midline back wounds to the extent that simple surgeries are insufficient for the task. In these cases, flap coverage is required.
Since the 1980s, myocutaneous flaps have become popular in the reconstruction of large soft tissue defects, including back wounds [ 2]. They provide well-vascularized bulky tissues for filling defects [ 2]. However, dissection, transposition, or rotation of the functioning muscles may lead to subsequent loss of donor site muscle function [ 2]. In addition, limited mobilization of the muscle flap towards the midline back wound can result in incomplete coverage [ 3]. While the free flap is another method for back reconstruction, it has the disadvantages of extended operation times and difficulty in changing postoperative positions to avoid the supine position [ 4]. In contrast, the perforator-based pedicled fasciocutaneous flap has many benefits for midline back wound coverage. These include the presence of reliable perforators, shorter surgery times, low donor site morbidity, availability of bilateral flaps for large-sized wound defects, and ease of postoperative position change [ 2]. In this study, we aimed to determine whether perforator-based pedicled fasciocutaneous flaps were effective in the coverage of midline back wounds mainly caused by spinal surgeries and pressure sores from paraplegia. In addition, to decrease discomfort of the donor sites, we attempted to close the donor sites primarily rather than using skin grafts.
Methods
Preoperative planning
Our algorithm for covering midline back wounds with perforator-based fasciocutaneous flaps is shown in Fig. 1. Preoperative evaluation of the patient was performed based on consultation with our department of plastic and reconstructive surgery. The patient's history was examined to determine the causes of the wounds, previously performed spine surgeries, and medical and physical conditions of the patients, including the feasibility of position changes. In addition, the aspects of the midline back wounds were examined, such as overall size and depth, cavity size, presence of active inflammation or growth of granulation tissues, and exposure of bone and/or metallic hardware. Wound culture for identification of patho-genic organisms (mainly bacteria) and susceptible antibiotics were examined, as were radiologic studies. Patients with medical comorbidities, such as severe cardiopulmonary disease, chronic debilitation, chronic renal failure, severe psychological problems, and those aged >80 years were excluded from this flap surgery.
Fig. 1.
Our algorithm for determining coverage of midline back wounds. 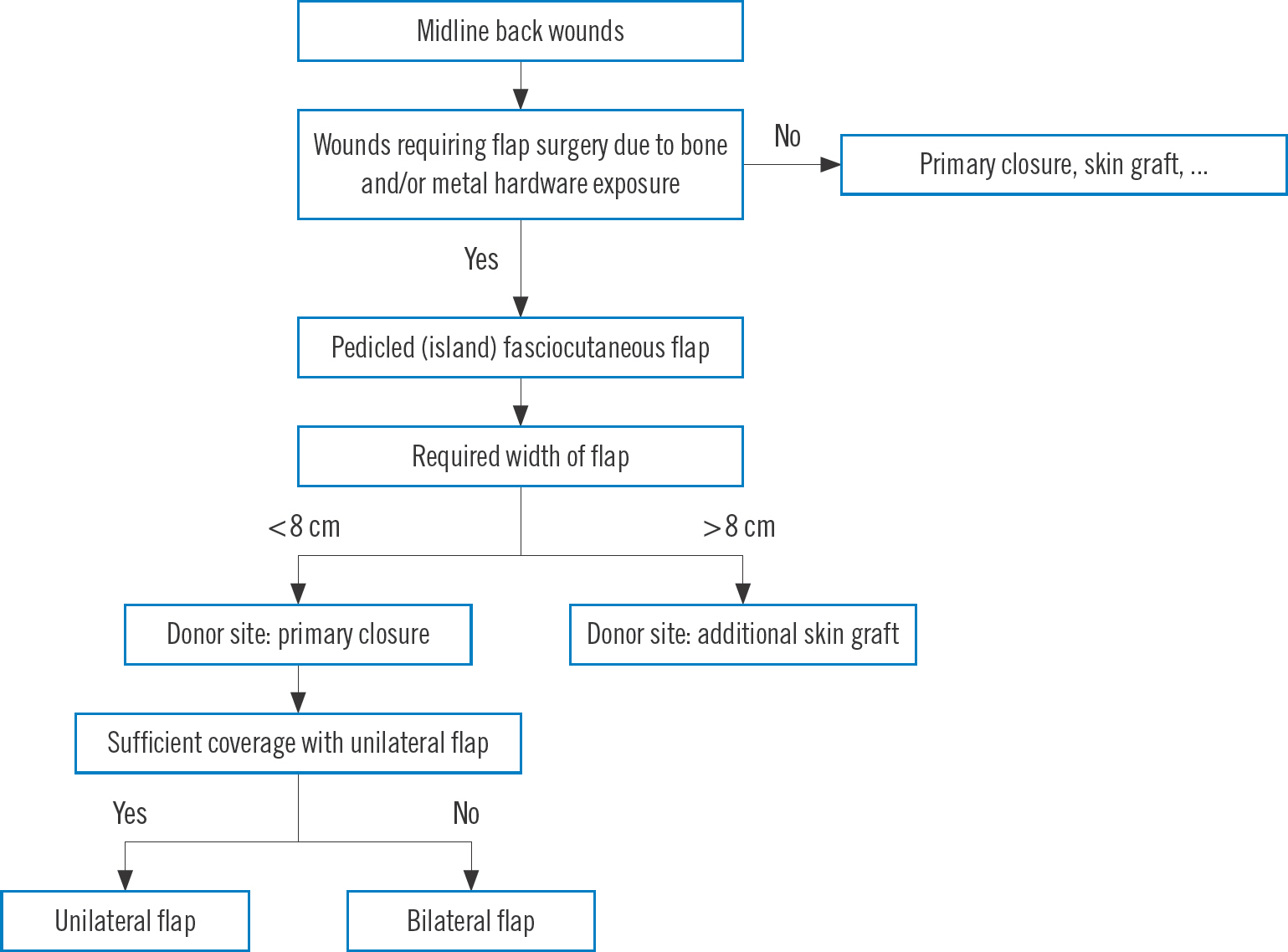
If the defect size was large (>5×10 cm) and bone and/or orthopedic metallic hardware were exposed, negative pressure wound therapy was applied for 2 or 3 weeks to reduce the wound size. To prepare for postoperative position adjustments, position changes between the supine, prone, and decubitus positions were exercised for 2 weeks before flap surgery.
The design of the flap, and identification of reliable perforators were both done for the purpose of facilitating flap coverage. The source vessels of the back perforators are from six types of source arteries from cephalad to caudad: dorsal intercostal artery, lumbar artery, lateral sacral artery, superior and inferior gluteal arteries, and parasacral artery ( Fig. 2). The perforators were mostly located at the immediate lateral site of the back midline or 4–8 cm lateral to the midline ( Fig. 3). Perforators were identified using a hand-held Doppler and mapped either near the midline of the back wounds or 4–8 cm away laterally from the midline of the back. The flap pedicle to be used for wound coverage was determined among such perforators. Using a marking pen, a preliminary design of the flap was made, taking into account post-debridement wound size increment ( Fig. 4). The designed skin flap was approximated with fingers to evaluate the possibility of primary closure. Within the designed flap, the perforators were identified again using a hand-held Doppler. After confirming the perforator vessels, the pivot point of the flap around the perforator vessel was tentatively determined, while the length and width of the flap were determined more precisely. An example of the flap design is shown in Fig. 5C. The final flap designs drawn with a marking pen are shown in Figs. 6A, 7C, and 8A.
Fig. 2.
The numerous perforators of the back from cephalad to caudad. 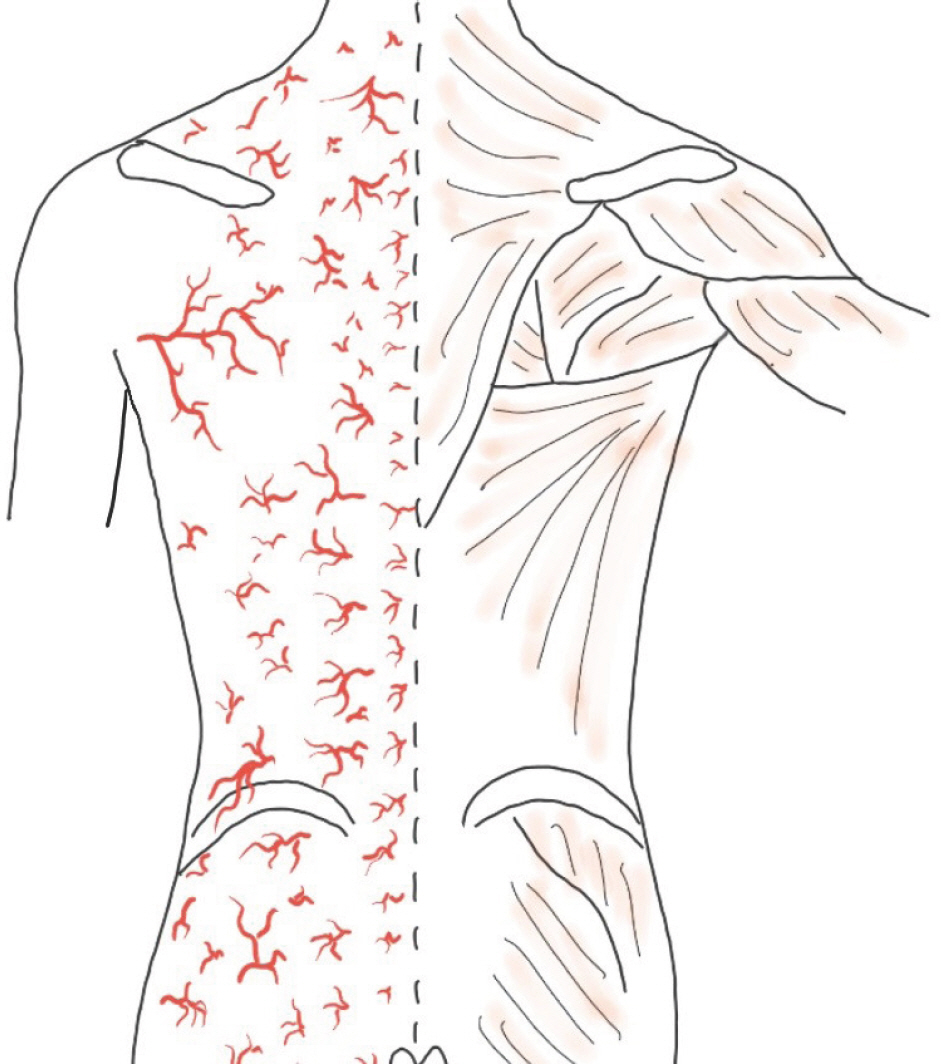
Fig. 3.
Medial and lateral cutaneous branches of the intercostal artery. The medial cutaneous branch is located lateral to the midline, while the lateral cutaneous branch is located 4–8 cm from the midline of the back (black arrows). 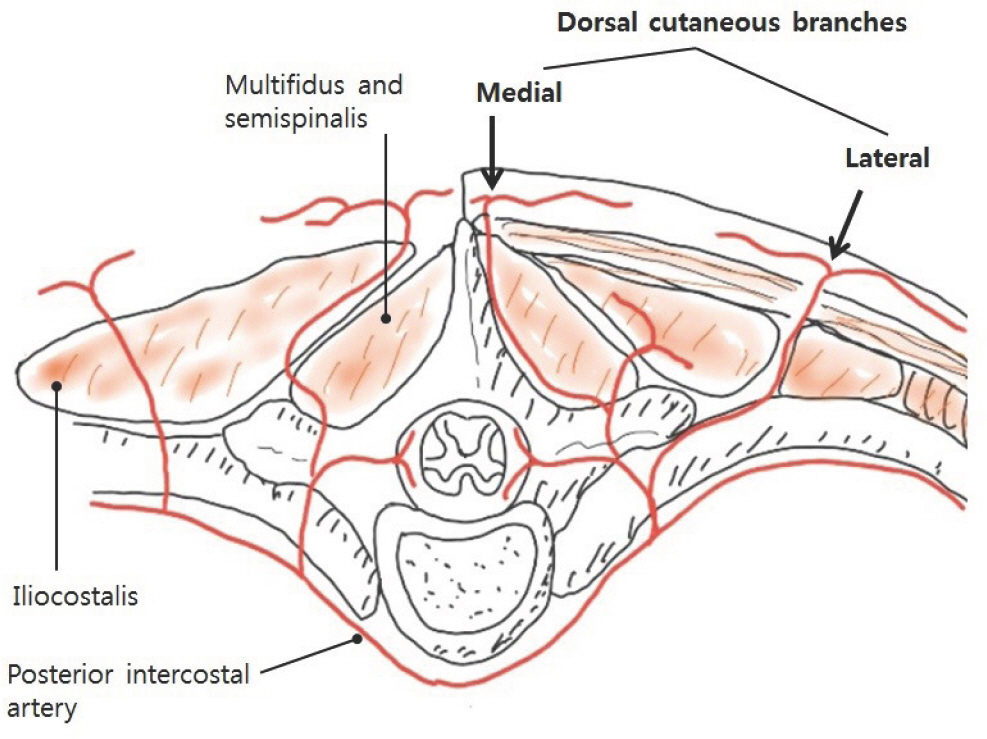
Fig. 4.
Sample back perforators and flap design. Our flap design method is shown in this illustration. The red points within the flap design indicate the locations of the perforator. The distal tip of the flap can be changed to cephalad to the pivot point, depending on the wound shape. 
Operative procedure
All patients were placed in prone positions under general anesthesia. The raw surfaces of the wounds were coated (dyed) with indigo carmine. Complete debridement and en bloc excision of the wound sac were performed. Periimplant sinus tracts were debrided as completely as possible along the metal to maintain bone stability. Cortical bone shaving or excision was also performed when spinal bony structure was exposed.
Preoperative design of the flap was repeated after debridement. Gauze cut in a shape identical to the flap was placed on the designed site of the flap, and then was translocated to the wound site at the pivot point. The authors adjusted the gauze to fit the wound surface in size, width, and depth before confirming the design.
Along the margins around the designed flap, 10–20 mL of 1% lidocaine with 1:200,000 epinephrine was infiltrated. An incision was made along the distal border (the portion farthest from the perforator vessels) of the predesigned flap. Flap dissection was then performed from the distal side (flap tip) to the proximal side (source vessels of the perforators) beneath the fasciocutaneous plane superficial to the back muscles. When the incision and dissection progressed to the proximal side (the pivot point), we carefully explored the perforators with either the naked eye or using a hand-held Doppler ( Figs. 5C, 7D, and 8C). Single or multiple perforators were identified and preserved in the flap pedicle. If perforators were not found intraoperatively at the previously detected site, though a rare occurrence, flap design was altered to the new perforator vessels at the pedicle site. Doppler tracing was repeated in the undissected area adjacent to the previously detected perforator vessels. After hearing the Doppler sound, further incision and dissection were performed around the new pedicle.
Fig. 5.
Bilateral dorsal thoracic intercostal artery island flaps (case 1). A 69-year-old woman was referred due to a thoracic midline wound after spinal surgery. (A) A 5×10-cm-sized wound was noted on the thoracic area with bone and metal exposure. (B) The radio-graph shows the metallic fixation hardware performed by an orthopedic spine surgeon. (C) Bilateral island flaps were dissected. The yellow surgical vessel loops indicate the perforators. The illustration shows the flap design and its transposition. The red dots indicate the location of the perforator. (D) The wound healed well without any complications. The gross photograph was taken at 12 months postoperatively. 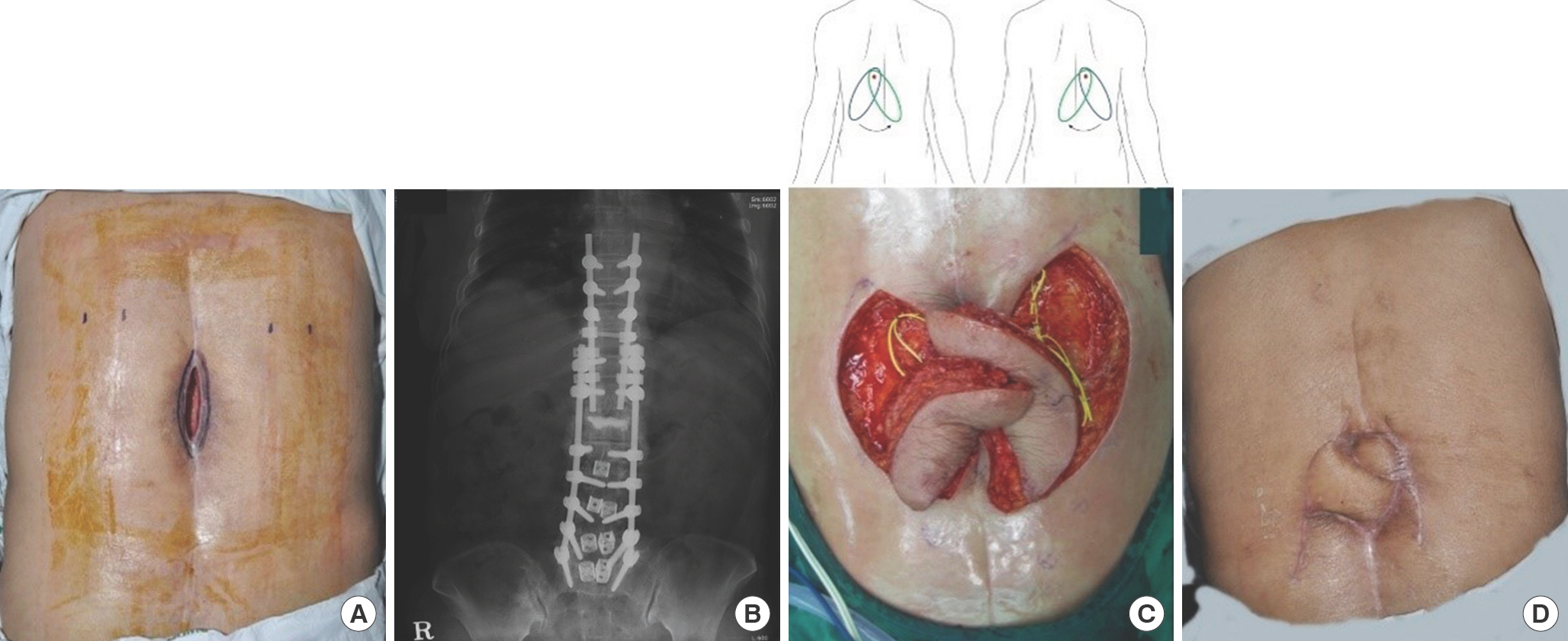
Fig. 6.
Subcutaneous pedicled fasciocutaneous flap for lumbar wound (case 2). (A, B) A 23-year-old man had undergone screw fixation at L4-L5-S1-ilium level that resulted in wound infection and hardware exposure. (C) A superior gluteal arterial perforator-based pedicled flap containing three perforators was elevated and transposed to cover the defect. The red surgical vessel loops indicate perforators from the superior gluteal artery. (D) The defect was covered successfully without complications. The gross photograph was taken at 35 months postoperatively. 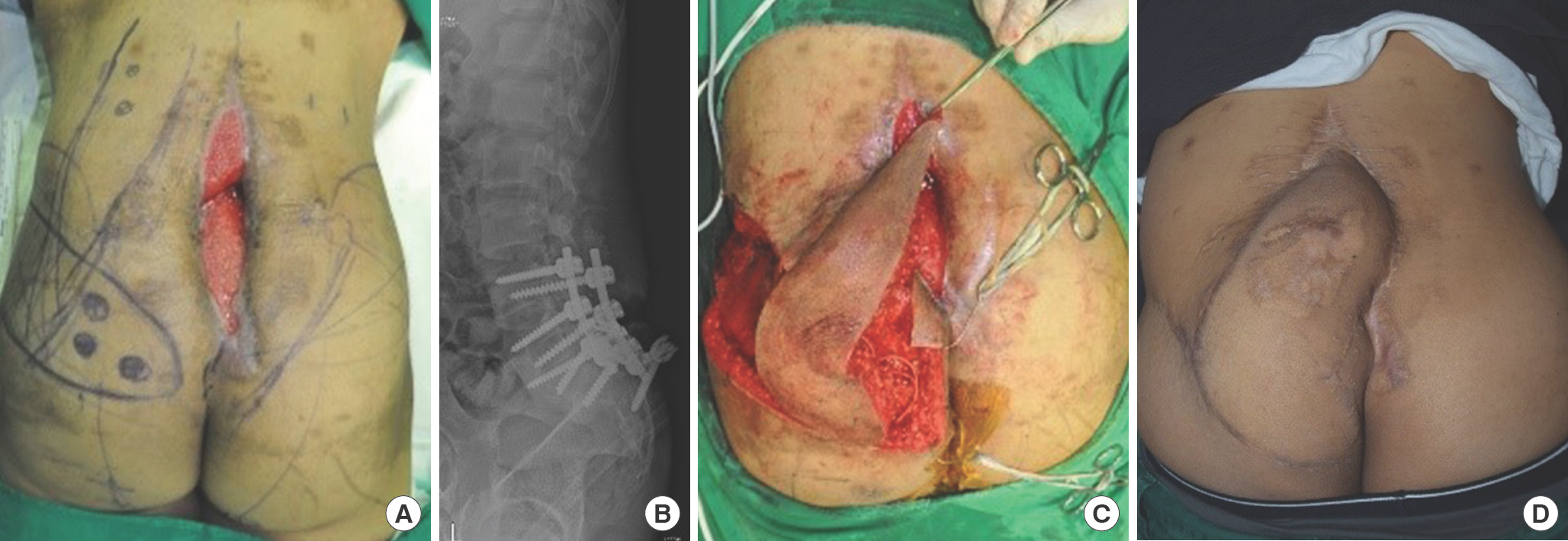
The dissected flap was transposed to the wound defect at the pivot point at which the perforator vessels emerged from the back muscle. We checked whether the flap fit the wound on all three dimensions and also examined the tension around the pivot point. The skin incision was not extended if there was little or no tension. If a wider rotation arc or more release of the vascular pedicle for tension release was necessary, further skin incision around the pedicle and dissection were performed. If the flap was larger or longer than the defect, the distal tip of the flap was excised or partially deepithelialized, and the deepithelialized dermal flap was used to fill the wound defect. Marginal dermal bleeding of the dissected flap was examined after transposition to the midline wound. Subsequently, the flap was inset and closed by each layer after the insertion of closedsuction drains ( Fig. 7F). The donor site was closed primarily. If tension was noted at the pivot point of the flap pedicle, a split-thickness skin graft (STSG) was used on the raw surface on the flap pedicle instead of primary closure to avoid choking of the perforator vessels.
Fig. 7.
Bilateral pedicled (peninsular) fasciocutaneous flaps for lumbar wound (case 4). (A) A 26-year-old man who had undergone orthopedic internal fixation on the lumbar spine and ilium after multiple spinal fractures at L3, 4, and 5 levels was referred due to wound infection and dehiscence. (B) This photograph shows metal structure for fixing lumbar spines and both iliac bones. (C) Inner hardware structure was exposed after repetitive debridement, with an 8×8-cm-sized lumbosacral midline defect. (D) The forceps tip shows the perforator from the left inferior gluteal artery. (E, F) Bilateral gluteal artery pedicled flaps (right side: 6×15 cm, left side: 8×20 cm) were elevated. The perforators were a superior gluteal artery perforator (right side), lateral sacral artery perforator, and inferior gluteal arterial perforator (left side). Marginal necrosis of 3×3-cm size had occurred on the flap tip of the left side; therefore, split-thickness skin graft was used. (G) Finally, the wound healed well. The gross photograph was taken at 9 months postoperatively. 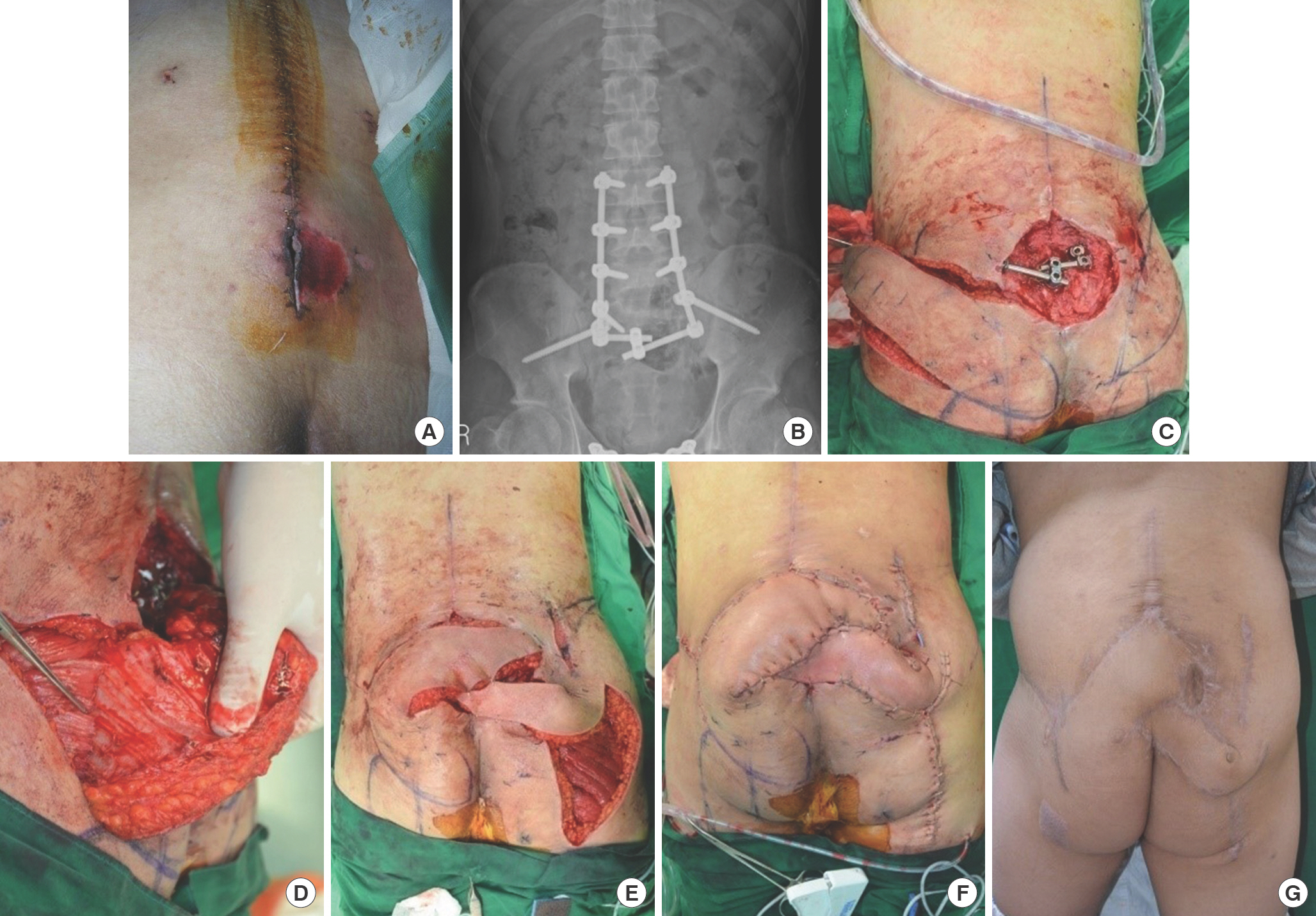
If a unilateral flap was not sufficient for coverage, a flap from the contralateral site was prepared using the same procedure. However, the contralateral flap did not have to be similar or symmetrical in shape, size, or perforator location to that of the previously dissected flap ( Fig. 7E). After wound closure, a gentle compressive dressing was performed with a window at the flap tip.
Postoperative course, complications of the wound and follow-up
To avoid pressure ischemia of the inset (transposed) flap, changes between the prone and alternate decubitus positions began 30 to 60 minutes after recovery of anesthesia. The patients were also allowed up to 30 minutes of supine positioning in between to decrease their discomfort. Care workers helped move the patients if they could not change their positions themselves. Two weeks after flap surgery, the patients were allowed to freely move into the supine position.
Gentle compressive wound dressing was performed daily for 10 days, and every other day afterwards. The wound, and particularly the flap tip was examined every 2 hours on postoperative day 1, and every 3 to 4 hours on postoperative days 2 and 3, through the window of the wound dressing. If flap congestion was noted at the flap tip during this period, several sutures were removed immediately. Two or three days after suture removal, if the flap tip color turned pink, the wound was re-approximated by metal stapling or suturing without tension. If the flap tip changed to necrotic dark, the necrotic portion was excised after postoperative day 7, and the wound was closed or a small-sized STSG was performed under local anesthesia. If wound infection was noted, drainage and betadine soaking dressings were performed daily. After the wound discharge decreased and the infection was controlled, the wound was closed. The closedsuction drain was removed when the drainage amount was less than 5 mL per day. Suture removal was performed on postoperative day 14. Correction of anemia and nutritional support were also performed postoperatively.
After the wound healed, the patient was discharged or transferred for medical or physical therapy. Subsequently, the wound was examined three times monthly. Further follow-up was provided every 3 to 6 months for 1 to 2 years, if necessary.
Written informed consent was obtained from all participants. This study was approved by the Institutional Review Board of Hallym University Sacred Heart Hospital (IRB No. 2020-04-001-001) and adhered to the ethical principles out-lined in the Declaration of Helsinki.
Results
Between May 2011 and November 2019, 13 patients (seven women, six men) with midline back wounds including skin and soft tissue defects were treated using our methods. The mean patient age was 56.5 years, and the mean hospitalization was 36.3 days. The follow-up period was 10 days to 35 months (mean: 9.9 months) ( Table 1).
Table 1.
Characteristics of patients
|
Case |
Sex/ age (yr) |
Cause of midline defect |
Area |
Wound size (cm) |
Flap size (cm) |
Perforator's number and origin |
STSG size on the flap pedicle (cm) |
Complications |
|
1 |
F/69 |
Wound infection after spinal surgery |
T spine |
5×10 |
8×12, 8×12 (B) |
1 DICAP, 1 LAP (R)
1 DICAP (L) |
None |
None |
|
2 |
M/23 |
Wound infection after spinal surgery |
L-S spine |
5×20 |
6×25 (L) |
3 SGAP |
3×4 |
None |
|
3 |
M/38 |
Infected epidermal cyst |
T spine |
5×10 |
6×15 (R) |
2 DICAP |
None |
None |
|
4 |
M/26 |
Wound infection after spinal surgery |
L-S spine |
6×8 |
6×15 (R),
8×20 (L) |
1 SGAP (R)
1 LSAP, 1 IGAP (L) |
None |
Flap tip necrosis |
|
5 |
F/47 |
Pressure sore |
S spine |
6×12 |
6×20 (R) |
1 SGAP |
None |
Dehiscence |
|
6 |
M/59 |
Pressure sore |
S spine |
6×7 |
8×15 (R) |
1 LSAP |
None |
None |
|
7 |
F/79 |
Wound dehiscence after spinal surgery |
L spine |
5×5 |
6×18 (L) |
3 LAP |
3×4 |
None |
|
8 |
F/82 |
Pressure sore |
Sacral spine |
2×3 |
5×17 (R) |
1 LSAP |
None |
None |
|
9 |
M/56 |
Pressure sore |
Sacro-coccyx spine |
3×4 |
6×17 (R) |
3 LSAP |
None |
Flap tip necrosis
Dehiscence |
|
10 |
F/71 |
Pressure sore |
Coccyx spine |
3×3 |
6×20 (L) |
2 SGAP |
None |
None |
|
11 |
F/80 |
Wound dehiscence after spinal surgery |
L spine |
5×6 |
8×15 (R) |
1 DICAP |
None |
Flap tip necrosis
Dehiscence |
|
12 |
F/75 |
Wound dehiscence after spinal surgery |
T spine |
2×2 |
6×12 (L) |
1 DICAP |
2.5×3 |
Flap tip necrosis
Dehiscence |
|
13 |
M/30 |
Coccygeal burn with pilonidal cyst infection |
Coccyx spine |
3×5 |
3×7 (L) |
1 Parasacral artery perforator |
None |
None |
The causes of the midline back wounds were wound dehiscence with bone and metallic hardware exposure after orthopedic spine surgery in six cases (two: thoracic, two: lumbosacral, and two: lumbar), and pressure sore in five cases. In these 11 patients, the wounds showed bone exposure due to necrosis of the soft tissues by wound infection. Another patient (case 3) had an infected epidermal cyst in the upper thoracic area, and the other paraplegic patient (case 13) had a pilonidal sinus combined with a third-degree thermal contact burn at the coccyx. In these two patients, the wound showed muscle (case 3) and ligament (case 13) exposures due to infection-induced necrosis of soft tissue.
The locations of the defects were in the thoracic spine area in three patients, lumbar area in two, lumbosacral area in two, sacral area in three, sacrococcygeal area in two, and coccygeal area in one. Preoperative negative pressure wound therapy was performed in 11 patients, excluding two cases of cyst infection (cases 3 and 13).
The wound sizes before debridement during flap surgery ranged from 2×2 cm to 5×20 cm. However, these wounds widened and deepened after debridement. The flap sizes of a unilateral site ranged from 3×7 cm to 8×20 cm. The width of a single unilateral flap was 8 cm at the maximum, because primary closure was difficult to achieve at a width wider than 8 cm. The average wound size before debridement at flap surgery was 35.6 cm2, and the mean flap size was 103.3 cm2. The average flap surgery time was 210 minutes.
All patients underwent a perforator-based pedicled fasciocutaneous flap (three patients with an island flap: cases 3, 5, 12; and 10 patients with a peninsular or subcutaneous pedicled flap: the others). Fifteen flaps were found to be elevated in 13 patients. A unilateral flap was used in 11 patients, and bilateral flaps were used in two patients (cases 1 and 4) ( Figs. 5, 7). In three cases (cases 2, 7 and 12), tension was noted at the pivot point of the flap pedicle and a small-sized STSG was used on the raw surface on the flap pedicle. The sizes of the skin grafts on the flap pedicle were 3×4 cm at the maximum. All the defects were closed without flap failure and the uptake of STSGs on the flap pedicles was favorable. The patient characteristics are listed in Table 1. All donor sites were closed primarily and healed without any complications. After flap surgery, distal tip venous congestion leading to small marginal necrosis was reported in four patients (cases 4, 9, 11, and 12). In one patient (case 4), partial necrosis of the flap tip was debrided and covered by a 3×4-cm-sized STSG, and the wound healed completely ( Fig. 7). Wound dehiscence occurred in four patients: two patients had underlying osteo-myelitis (cases 11 and 12), and two other patients could not change postoperative positions freely due to general weakness and paraplegia caused by spinal cord injury (cases 5 and 9). In these patients, after 2 or 3 weeks of betadine-soaked gauze dressing, the dehisced wounds were re-closed or covered with STSG. Consequently, two patients healed completely (cases 5 and 12), but one patient was lost to follow-up in the outpatient department, and the other patient was transferred to another hospital (cases 9 and 11). One patient experienced a small wound 12 months after flap surgery (case 4). A small sinus (diameter, 1 cm) was noted on the margin of the wound on the right side of the lumbosacral area. The wound was explored under general anesthesia. A 2×2-cm-sized sinus cavity was noted along the metal tract, and complete debridement and reclosing were performed. The wound healed well and there was no recurrence after 6 months of resurgery ( Fig. 7).
Discussion
The perforator flap was introduced in the late 1980s by Kroll and Rosenfield [ 5]. Recently the perforator flap has been used more frequently in back reconstruction [ 2]. In addition to the paraspinal muscle flap or free flap, the perforator-based pedicled flap has been used in midline back reconstruction [ 6]. The perforator-based pedicled fasciocutaneous flap has the advantages of reliable vascular supply and ease of primary closure of the donor site [ 2, 7]. Furthermore, fasciocutaneous flap surgery does not require muscle dissection, leads to less blood loss and no damage to the functioning muscles. Therefore, patients who undergo fasciocutaneous flap complain of less pain, and early mobilization (position change) is possible because there is no functional deficit at the donor site [ 2]. In addition, through animal studies, Guerra et al. [ 8] showed that there was no significant difference between latissimus dorsi muscle flaps and fasciocutaneous perforator flaps in local infection control. The free flap, which is more invasive than the perforator-based pedicled (island) flap, provides bulky and healthy soft tissue. However, for its success, sufficient recipient vessels and longer surgery times are needed [ 4]. Postoperatively, it is cum-bersome for patients to change their position to avoid flap compression when in the supine position. de Weerd et al. [ 1] reported that early postoperative position changes might prevent flap necrosis and wound dehiscence. In patients with early mobilization on postoperative day 2, all flaps (10 flaps) survived except one flap with marginal necrosis in their report. Additional perforator-based pedicled fasciocutaneous flaps are easily designed at the contralateral side, if bilateral flaps for larger defects are necessary. In our study, several large wound defects (>45 cm 2 after debridement) in the midline area were covered by bilateral perforator-based pedicled flaps (cases 1 and 4) ( Figs. 5, 7). The flap design by the authors was not always based on the perforasome territory. The most important factor in deciding whether to use a unilateral flap or bilateral flap was the feasibility of donor site closure. Adjacent perforasome territories can be included in one large perforator flap because the flaps of the different perforasome territories could survive by connecting vessels ( Fig. 6) [ 9]. It is debatable how large a flap can survive on a single perforator. Minabe and Harii [ 10] elevated a flap sized 7×18 cm based on a single perforator without tip necrosis. In our cases, a bulky 6×20-cm flap with a single perforator survived without marginal necrosis (case 5) ( Fig. 8). It is observed that, if two or more perforators are in the flap pedicle, the flap perfusion is safer. In such cases, a sufficiently large flap can be used because of its rich vascularity due to the presence of many perforators. However, in these cases, the rotation arc is narrow, and flap mobility is limited. Keles et al. [ 11] reported that flap perfusion was compromised even when multiple perforators were in the flap pedicle in an animal study. This might be caused by pedicle kinking due to the wide rotation arc [ 12]. Therefore, avoiding tension at the flap pedicle is important for flap survival.
Fig. 8.
Parasacral artery island flap for sacral sore (case 5). (A) A 47-year-old woman with paraplegia was referred for a sacral pressure sore with paraplegia. (B, C) After debridement, a superior gluteal arterial perforator island flap was elevated and transposed by 180° of rotation. The forceps tip indicates the perforator. (D) The wound healed well. The gross photograph was taken at 2 months postoperatively. 
In our study, postoperative minor complication rates such as partial flap tip necrosis and wound dehiscence occurred in five patients (38.5%), which was higher than that in other reports. In other studies which used similar methods, the rates of postoperative minor complications were 5.25%–19.1%. However, major complications of total flap necrosis did not occur in our study, while in other studies there were major complications (1.5%–5%) [ 6, 13]. In our study, the etiology of the wound was mainly spinal surgery and paraplegia (92.3%), in contrast to other studies (50%–70%). The other main causes of midline back wounds were tumors. Therefore, we suspected that minor complications occurred more frequently in patients who had undergone previous spinal surgery or paraplegia than in those who underwent tumor surgery. However, this hypothesis requires confirmation with further studies. Most authors prefer flaps that fit right into the wound defects. However, based on the author's personal experience, a flap slightly larger (up to 105% of the wound size) than the exact size of the wound site was also acceptable, as it provides a reliable margin in the case of minor postoperative complications Considering the possibility of minor postoperative complications such as infection, sinus formation, and the possibility of hardware removal due to instability of the bony structure, a flap even slightly larger was also recommendable. One patient (case 4) presented with a sinus tract at the previous surgical site 32 months after bilateral superior gluteal arterial perforator flaps had been inserted. Turbid discharge was drained from the lumbosacral area. The sinus tract reached an internally-fixed metal structure in the vertebral bone. After irrigation, the wound was explored, and the sinus tract was completely excised. The wound was closed using direct closure without tension. Fortunately, skin and soft tissue insufficiency did not occur because the flap had been slightly oversize. Consequently, a new flap or skin grafting was not necessary and the wound healed well.
The disadvantage of the perforator-based peninsular or subcutaneous pedicled flap, but not the island flap, is bulkiness at the pivot point, which may require a secondary procedure such as liposuction or defatting. During long-term follow-up, four patients complained of discomfort in the supine position due to bulkiness at the pedicle site after flap surgery. In one patient (case 2), defatting was performed 12 months postoperatively under general anesthesia ( Fig. 6). Another disadvantage is that flap design changes are necessary during surgery if the perforator is extremely small or absent; however, such cases are rare. The limitations of this study are as follows: there were less cases included than of those in recent reports on back and midline back reconstruction using perforator flaps [ 6, 13]. Cases with very large wound (wider than 8 cm) coverage were not included. The width of a single flap was also less than 8 cm for the primary closure of the donor site. For large wound (wider than 8 cm) coverage, the flap size must be wider, making primary closure difficult. In such cases, a skin graft is necessary at the donor site. However, as shown in our study, primary closures of the donor sites may be also possible using bilateral pedicled flaps for large wounds.
In conclusion, the perforator-based pedicled fasciocutaneous flap can be a useful option in coverage of midline back wounds with skin and soft tissue defects from various causes. The perforator-based pedicled fasciocutaneous flap has the advantages of reliable perforators, availability of bilateral flaps, preservation of functioning muscles, and ease of early position changes.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
1. de Weerd L, Solberg TK, Weum S. Closure of complex posterior midline defects after spinal surgery with sensate midline-based perforator flaps and the long-term results. Spine (Phila Pa 1976) 2015;40:E1233-8.   2. Geddes CR, Morris SF, Neligan PC. Perforator flaps: evolution, classification, and applications. Ann Plast Surg 2003;50:90-9.   3. Wilhelmi BJ, Snyder N, Colquhoun T, et al. Bipedicle para-spinous muscle flaps for spinal wound closure: an anatomic and clinical study. Plast Reconstr Surg 2000;106:1305-11.   4. Hung SJ, Chen HC, Wei FC. Free flaps for reconstruction of the lower back and sacral area. Microsurgery 2000;20:72-6.   5. Kroll SS, Rosenfield L. Perforator-based flaps for low posterior midline defects. Plast Reconstr Surg 1988;81:561-6.   7. Cormack GC, Lamberty BGH. The arterial anatomy of skin flaps. 2nd ed. Edinburgh: Churchill Livingstone; 1994.
8. Guerra AB, Gill PS, Trahan CG, et al. Comparison of bacterial inoculation and transcutaneous oxygen tension in the rabbit S1 perforator and latissimus dorsi musculocutaneous flaps. J Reconstr Microsurg 2005;21:137-43.   9. Saint-Cyr M, Wong C, Schaverien M, et al. The perforasome theory: vascular anatomy and clinical implications. Plast Reconstr Surg 2009;124:1529-44.   10. Minabe T, Harii K. Dorsal intercostal artery perforator flap: anatomical study and clinical applications. Plast Reconstr Surg 2007;120:681-9.   11. Keles MK, Demir A, Kucuker I, et al. The effect of twisting on single and double based perforator flap viability: an experimental study in rats. Microsurgery 2014;34:464-9.   12. Lee HJ, Lim SY, Pyon JK, et al. The influence of pedicle tension and twist on perforator flap viability in rats. J Reconstr Microsurg 2011;27:433-8.   13. Hernekamp JF, Cordts T, Kremer T, et al. Perforator-based flaps for defect reconstruction of the posterior trunk. Ann Plast Surg 2021;86:72-7.  
|
|























