Introduction
Myelodysplastic syndromes (MDS) are caused by malignant hematopoietic stem cells [1]. Though peripheral blood cytopenia with dysplastic changes in hypercellular bone marrow are hallmarks of MDS, its clinical manifestations are not distinctive. Some patients may present with variable symptoms including chronic fatigue, petechiae, infections, and other complications of cytopenia, while others may be asymptomatic [2,3]. Herein, we present a case of unexplained recurrent cellulitis with no other specific symptoms; the patient was finally diagnosed with MDS during his second hospital stay for treatment of recurrent cellulitis. Informed consent for use of photographs was obtained from the patient.
Case
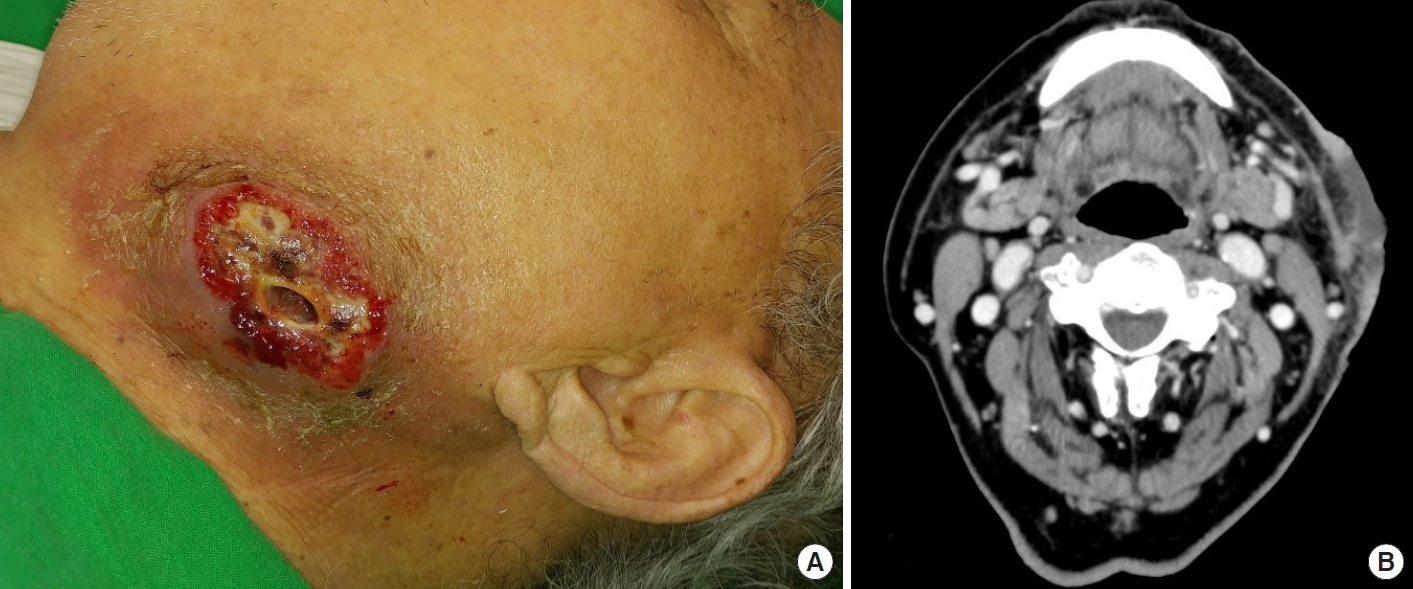
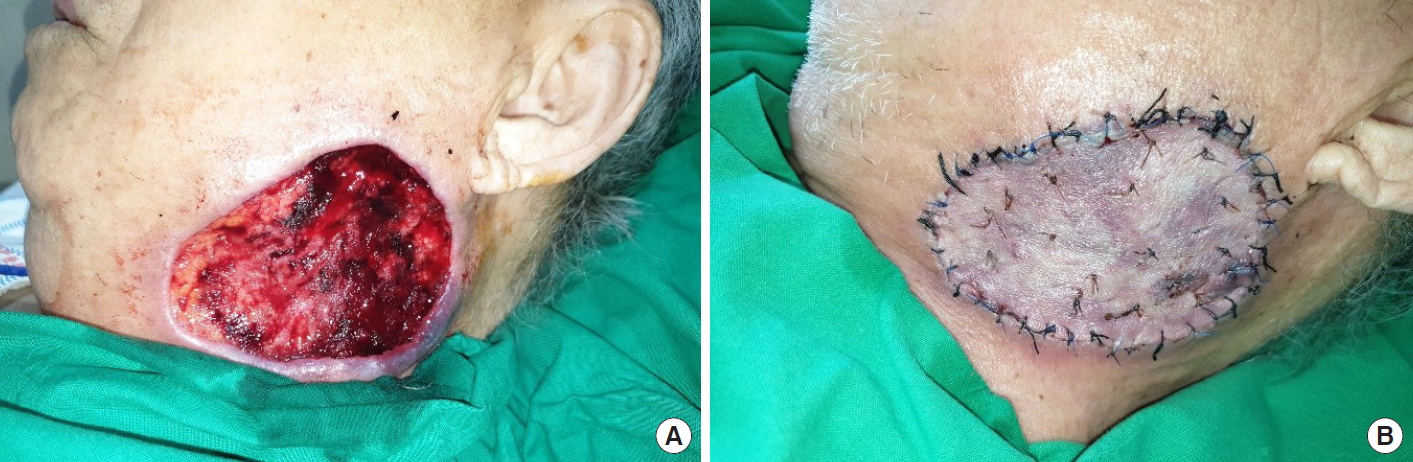
An 84-year-old male patient was referred for recurrent swelling and pus-like discharge over the left side of his neck for 2 weeks prior to presentation. The patient had been diagnosed with cellulitis and abscess formation and had undergone repeated incision and drainage at another facility before visiting us. The initial physical examination revealed a 5×3 cm, severely painful, suppurative ulcer in the left submandibular region (Fig. 1A). Enhanced computed tomography (CT) showed an enhanced skin thickening 6 cm in diameter with central necrosis in the left submandibular area, implying cellulitis or malignancy of the skin. There was no visible abscess formation or evidence of cervical lymphadenopathy (Fig. 1B). The patient underwent debridement and negative pressure wound therapy, with bacterial culture and histopathologic examinations (Fig. 2A). Initial lab findings showed results suggesting pancytopenia; his white blood cell count was 4.4×103/mm3, hemoglobin was 7.8 g/dL, and platelet count was 128×103/mm3. There was no febrile episode. Methicillin-resistant Staphylococcus aureus (MRSA) was identified in the wound culture. Histologic findings showed acute necrotizing suppurative inflammation (Fig. 3).
After administration of intravenous antibiotics, the wound improved and stabilized with decreased discharge. The defect was reconstructed using a split-thickness skin graft (Fig. 2B). During hospitalization, the patient was referred to the hematooncology department for evaluation of pancytopenia. On gastrofiberscopy, there was no evidence of gastrointestinal bleeding. The patient’s neutropenia was believed to be drug-induced, possibly due to antibiotics. The patient was managed conservatively and discharged with a completely healed wound.
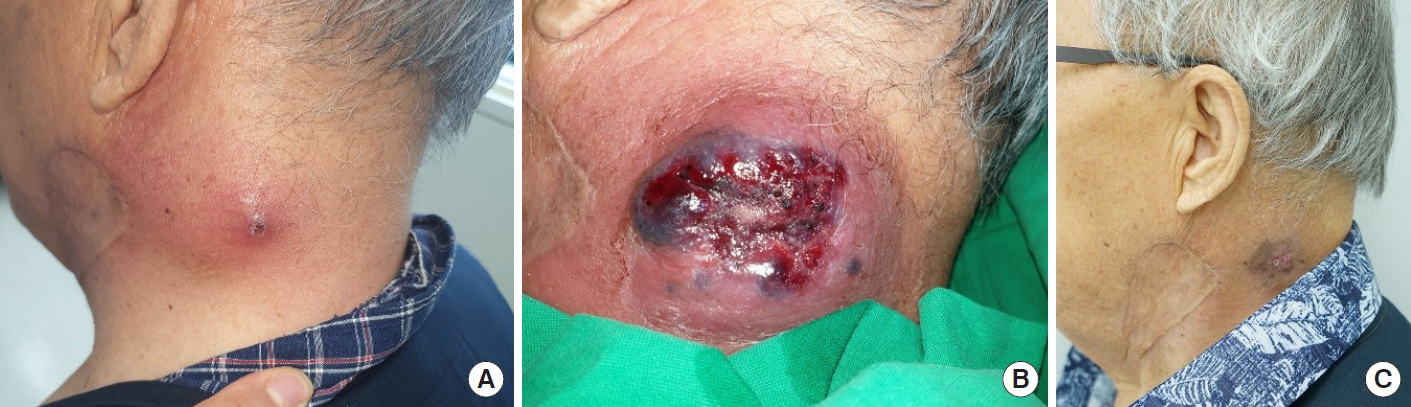
Two months later, the patient returned with a localized inflammation on the neck adjacent to where he had the skin graft. He presented with pain similar to the past episode, ulcerative lesion, and fever (Fig. 4A and B). The initial laboratory findings showed pancytopenia and elevated C-reactive protein (CRP) (white blood cell, 4.4×103/μL; hemoglobin, 9.6 g/dL; platelet, 128×103/μL; CRP, 3.51 mg/dL). Enhanced neck CT showed findings suggestive of cellulitis at the left posterior neck. Histopathologic findings demonstrated acute suppurative inflammation, and MRSA was cultured again. After administration of intravenous vancomycin to target the MRSA, the ulcerative lesion improved. However, the follow-up laboratory findings showed aggravated pancytopenia and the patient experienced persistent high fever. Considering the history of pancytopenia, the patient was sent for a re-consultation and further evaluation at the hemato-oncology department. A bone marrow biopsy was performed, and the patient was finally diagnosed with MDS (Fig. 5).
During the second hospitalization, the patient was managed only with conservative dressing and intravenous antibiotics. The patient was discharged with improvement in cellulitis and treated at the hemato-oncology department (Fig. 4C). The cause of the unexplained recurrent cellulitis in this case was MDS.
Discussion
MDS are clonal disorders of malignant hematopoietic stem cells characterized by dysplastic and ineffective hematopoiesis, resulting in a high risk of transformation to acute myeloid leukemia [1,2]. They occur most commonly in older adults [3,4]. MDS have been associated with benzene derivatives, radiation, tobacco, chemotherapy, and inherited genetic abnormalities [2,5].
Patients with MDS may experience prolonged periods of fatigue or exertional dyspnea over 6–12 months, recurrent infections, petechiae, and other signs of bleeding. However, many patients are asymptomatic at diagnosis, and abnormalities in routine blood counts such as anemia, neutropenia, and thrombocytopenia are usually found first [6].
The diagnosis of MDS is based on a peripheral blood smear and bone marrow biopsy with a suggestive clinical context. Significant dysplasia in erythroid precursors, granulocytes, or megakaryocytes is observed on the blood smear or bone marrow examination [7]. Cytogenetic abnormalities can be the evidence of MDS in cases with no morphologic evidence of dysplasia.
The high risk of infection in MDS patients is well known and is considered a significant cause of morbidity and mortality [2,8]. Most infectious complications originate from bacteria, and cellulitis accounts for approximately 30% of infectious complications [8]. The infections are mainly attributed to the quantitative and qualitative defects in neutrophils. Guidelines for the treatment of infection in MDS patients have not yet been established. Some studies have shown that granulocyte colony-stimulating factor improves neutropenia in 30% to 70% of neutropenic MDS cases; however, its efficacy in preventing infection has not been proven [8]. Meanwhile, antibacterial prophylaxis may increase bacterial resistance, but the benefits and risks of prophylactic antimicrobials are not confirmed. Use of antibacterial prophylaxis is believed to be reasonable in patients who start therapy with demethylating agents and in those with a neutrophil count under 500×109/L [8]. Patients with MDS should be aware of the risk of infection. In case of febrile neutropenia, patients should be admitted with immediate tests, including blood cultures. Immediate broad-spectrum empirical antibiotics are warranted in such patients, and the clinical presentation should be considered when choosing the antibiotics.
This case is significant as our patient had no infections other than the recurrent cellulitis and his routine blood counts were at the limit of the normal ranges. While the high risk of infection in MDS patients is well-characterized, diagnosing MDS as the underlying cause of recurrent cellulitis is challenging, especially since cellulitis has various causes and often recurs, and MDS themselves are rare diseases [4,9]. Since many patients with MDS are asymptomatic and the clinical manifestations are not specific, an abnormal routine blood count is important when suspecting MDS. The initial laboratory findings of this patient suggested pancytopenia; however, his blood count was at the limit of the normal range. Patients with borderline laboratory results are likely to be misdiagnosed. Indeed, the hemato-oncologist suspected that the mild neutropenia and thrombocytopenia was possibly due to inflammation. The history of intermittent hematochezia could explain his low hemoglobin level. Furthermore, the lack of other symptoms or infections mean that there was no evidence of immunodeficiency. Thus, the patient was thought to have an uncontrolled infection of the skin lesion.
In cases of recurrent cellulitis with findings suggesting pancytopenia, predisposing factors that could cause immunosuppression should be considered. Hematologic malignancies, including MDS or acute leukemia, can cause pancytopenia through bone marrow infiltration. The use of cytotoxic drugs, underlying autoimmune diseases, poor nutritional status, and viral infections such as human immunodeficiency virus (HIV) are all possible factors associated with bone marrow failure [10]. Diabetes mellitus is a common predisposing factor that can cause secondary immunodeficiency. The patient in this case had no medical history of cytotoxic drugs, no underlying diabetes mellitus, and no autoimmune diseases. Tests for viral infection, including HIV and hepatitis, showed negative results. There were no problems with his nutritional status. Through bone marrow biopsy, we found that his pancytopenia could be attributed to MDS.
Sweet syndrome is a disease that can mimic cellulitis in patients with MDS [11]. Differential diagnosis is necessary because Sweet syndrome responds well to systemic glucocorticoids. In this case, Sweet syndrome could not be histologically ruled out because neutrophilic dermatosis without clear evidence of leukocytoclastic vasculitis was seen. However, the skin lesion was far from the erythematous plaques seen in Sweet syndrome [12].
This case shows that recurrent cellulitis that is difficult to resolve may not simply be a case of uncontrolled infection. If the patient’s laboratory findings suggest pancytopenia, the recurrent infection could be attributed to systemic reasons, such as MDS.